Summary: Bcl6 acts as a driver of neurogenic transition by directly silencing a selection of genes that belong to multiple extrinsic pathways promoting self-renewal. Bcl6 is only expressed in specific subsets of neurons and progenitor cells during brain development.
Source: VIB
Researchers led by Pierre Vanderhaeghen and Jérôme Bonnefont (VIB-KU Leuven and ULB) have unraveled a new mechanism controlling the switch between growth and differentiation of neural stem cells during brain development. They discovered a specific factor that makes stem cells ‘deaf’ to proliferative signals, which in turn causes them to differentiate into neurons and shape the marvelous complexity of our brain. The findings, published in this week’s edition of Neuron, shed new light on our understanding of brain developmental processes and have important implications for stem cell biology.
The brain is an incredibly complex organ consisting of billions of cells with a diverse range of functions. The mechanisms that orchestrate the formation of this intricate network during development have kept neuroscientists awake for decades.
One such neuroscientist is Prof. Pierre Vanderhaeghen (VIB-KU Leuven) whose team studies the development of the brain cortex, the outer layer of neuronal tissue that contributes in an essential way to who we are, as a species and as individuals.
“During neural development, a complex cocktail of signals determines the fate of neuronal progenitor cells,” explains Vanderhaeghen.
“These stem cells receive many different ‘proliferative’ signals that instruct them to keep on dividing, generating more and more cells for the growing brain, but at some point they also need to stop doing this and differentiate. In other words, they need to specialize to become a specific type of brain cell.”
Turning deaf at the right time to mature into a nerve cell
Vanderhaeghen’s team set out to understand how this switch between growth and differentiation is regulated and identified a molecular factor, called Bcl6, that essentially makes progenitor cells “deaf” for the proliferative signals that tell them to keep on dividing, thereby ensuring that differentiation occurs efficiently.
Jérôme Bonnefont, a postdoctoral researcher in Vanderhaeghen’s lab, explains: “We used an extensive set of genomic and cellular tools and found that a protein called Bcl6 acts as a global repressor of a repertoire of signaling components and pathways that are known to promote self-renewal. Since Bcl6 is expressed only in specific subsets of progenitors and neurons during brain development, it allows for the precise fine-tuning of brain developmental processes.”
Fate transition, stem cells, and cancer
Vanderhaeghen is enthusiastic about the findings: “These results provide important insight into the molecular logic of so-called neurogenic conversion. Thanks to this ingenious switch, differentiation can occur in a robust way despite the presence of many, and sometimes even contradictory, extrinsic cues.”
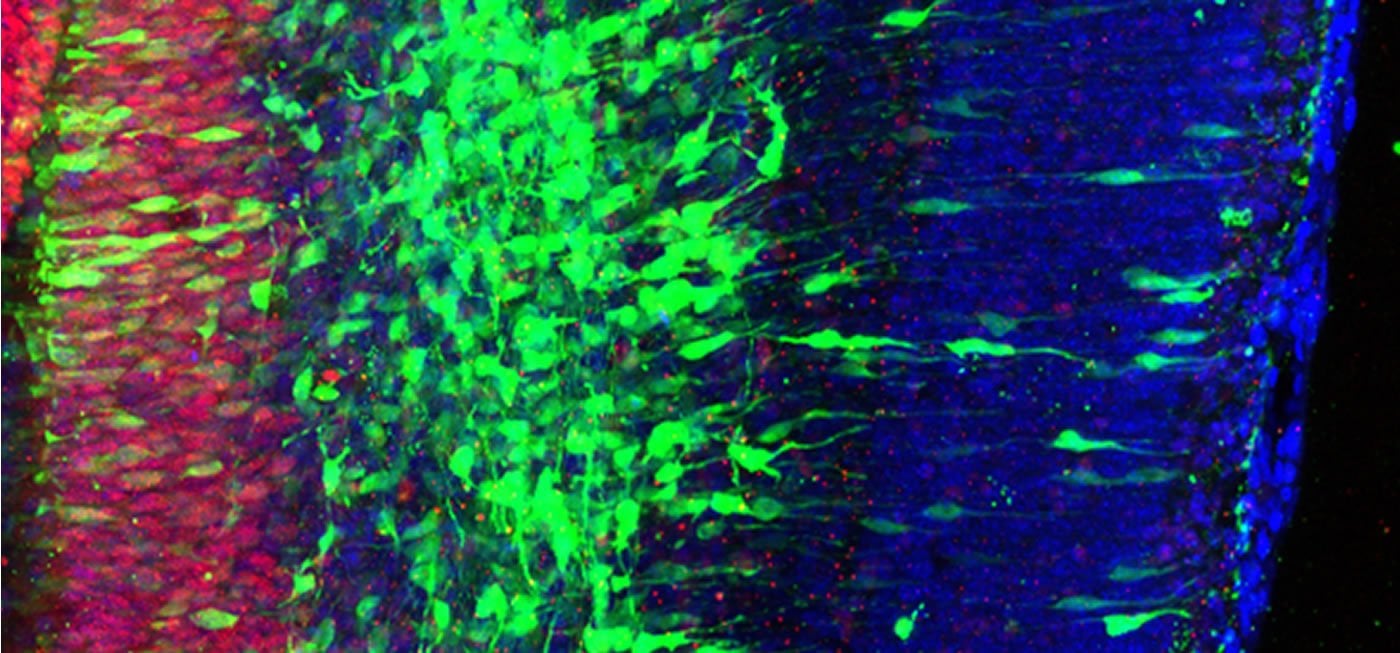
“We made this discovery focusing on neural stem cells, but I would predict that similar factors act in many stem cells in the embryo and even in adults to ensure proper differentiation,” he continues. “This may be also important in the context of cancer biology, since stem cells and cancer cells usually respond to the same proliferative cues that are precisely inhibited by Bcl6.”
Future work should determine whether and how other repressors in other parts of the nervous system and body can modulate responsiveness to extrinsic cues in a similar way. This will teach us more about differentiation, not only during development, but also beyond in the adult brain and in cancer cells.
Funding: This work was the result of a collaboration between the VIB KU Leuven, ULB, Belgium, and the Crick Institute, UK. It was funded by the European Research Council (ERC Adv Grant GENDEVOCORTEX), the Belgian FRS/FNRS, the VIB, the Queen Elizabeth Medical Foundation, the Interuniversity Attraction Poles Program (IUAP), the WELBIO Program of the Walloon Region, the AXA Research Fund, the Fondation ULB, the ERA-net ‘Microkin’, and EMBO.
Source:
VIB
Media Contacts:
Emily Velasco – VIB
Image Source:
The image is credited to VIB.
Original Research: Open access
“Cortical neurogenesis requires Bcl6-mediated transcriptional repression of multiple self-renewal-promoting extrinsic pathways”. Bonnefont et al.
Neuron. doi:10.1016/j.neuron.2019.06.027
Abstract
Cortical neurogenesis requires Bcl6-mediated transcriptional repression of multiple self-renewal-promoting extrinsic pathways
Highlights
• Bcl6 ensures robust neurogenesis by repressing major extrinsic self-renewal pathways
• Bcl6 inhibits the Notch, Wnt, SHH, and FGF signaling pathways at multiple levels
• Bcl6 represses transcription through Sirt1 recruitment and histone deacetylation
Summary
During neurogenesis, progenitors switch from self-renewal to differentiation through the interplay of intrinsic and extrinsic cues, but how these are integrated remains poorly understood. Here, we combine whole-genome transcriptional and epigenetic analyses with in vivo functional studies to demonstrate that Bcl6, a transcriptional repressor previously reported to promote cortical neurogenesis, acts as a driver of the neurogenic transition through direct silencing of a selective repertoire of genes belonging to multiple extrinsic pathways promoting self-renewal, most strikingly the Wnt pathway. At the molecular level, Bcl6 represses its targets through Sirt1 recruitment followed by histone deacetylation. Our data identify a molecular logic by which a single cell-intrinsic factor represses multiple extrinsic pathways that favor self-renewal, thereby ensuring robustness of neuronal fate transition.






