Summary: Researchers report proteins produced by gut bacteria may cause protein misfolding in the brain and cerebral inflammation.
Source: University of Louisville.
Research at UofL funded by The Michael J. Fox Foundation shows proteins produced by gut bacteria may cause misfolding of brain proteins and cerebral inflammation.
Alzheimer’s disease (AD), Parkinson’s disease (PD) and Amyotrophic Lateral Sclerosis (ALS) are all characterized by clumped, misfolded proteins and inflammation in the brain. In more than 90 percent of cases, physicians and scientists do not know what causes these processes to occur.
Robert P. Friedland, M.D., the Mason C. and Mary D. Rudd Endowed Chair and Professor of Neurology at the University of Louisville School of Medicine, and a team of researchers have discovered that these processes may be triggered by proteins made by our gut bacteria (the microbiota). Their research has revealed that exposure to bacterial proteins called amyloid that have structural similarity to brain proteins leads to an increase in clumping of the protein alpha-synuclein in the brain. Aggregates, or clumps, of misfolded alpha-synuclein and related amyloid proteins are seen in the brains of patients with the neurodegenerative diseases AD, PD and ALS.
Alpha-synuclein (AS) is a protein normally produced by neurons in the brain. In both PD and AD, alpha-synuclein is aggregated in a clumped form called amyloid, causing damage to neurons. Friedland has hypothesized that similarly clumped proteins produced by bacteria in the gut cause brain proteins to misfold via a mechanism called cross-seeding, leading to the deposition of aggregated brain proteins. He also proposed that amyloid proteins produced by the microbiota cause priming of immune cells in the gut, resulting in enhanced inflammation in the brain.
The research, which was supported by The Michael J. Fox Foundation, involved the administration of bacterial strains of E. coli that produce the bacterial amyloid protein curli to rats. Control animals were given identical bacteria that lacked the ability to make the bacterial amyloid protein. The rats fed the curli-producing organisms showed increased levels of AS in the intestines and the brain and increased cerebral AS aggregation, compared with rats who were exposed to E. coli that did not produce the bacterial amyloid protein. The curli-exposed rats also showed enhanced cerebral inflammation.
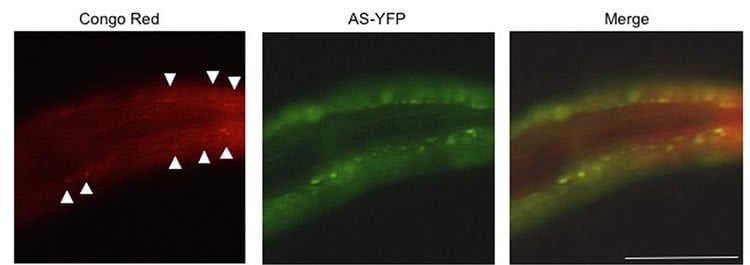
Similar findings were noted in a related experiment in which nematodes (Caenorhabditis elegans) that were fed curli-producing E. coli also showed increased levels of AS aggregates, compared with nematodes not exposed to the bacterial amyloid. A research group led by neuroscientist Shu G. Chen, Ph.D., of Case Western Reserve University, performed this collaborative study.
This new understanding of the potential role of gut bacteria in neurodegeneration could bring researchers closer to uncovering the factors responsible for initiating these diseases and ultimately developing preventive and therapeutic measures.
“These new studies in two different animals show that proteins made by bacteria harbored in the gut may be an initiating factor in the disease process of Alzheimer’s disease, Parkinson’s disease and ALS,” Friedland said. “This is important because most cases of these diseases are not caused by genes, and the gut is our most important environmental exposure. In addition, we have many potential therapeutic options to influence the bacterial populations in the nose, mouth and gut.”
“We are pursuing studies in humans and animals to further evaluate the mechanisms of the effects we have observed and are exploring the potential for the development of preventive and therapeutic strategies,” Friedland said.
Friedland is the corresponding author of the article, Exposure to the functional bacterial amyloid protein curli enhances alpha-synuclein aggregation in aged Fischer 344 rats and Caenorhabditis elegans, published online Oct. 6 in Scientific Reports, a journal of the Nature Publishing Group. UofL researchers involved in the publication in addition to Friedland include Vilius Stribinskis, Ph.D., Madhavi J. Rane, Ph.D., Donald Demuth, Ph.D., Evelyne Gozal, Ph.D., Andrew M. Roberts, Ph.D., Rekha Jagadapillai, Ruolan Liu, M.D., Ph.D., and Richard Kerber, Ph.D. Additional contributors on the publication include Eliezer Masliah, M.D., Ph.D. of the University of California San Diego.
Funding: This work supports recent studies indicating that the microbiota may have a role in disease processes in age-related brain degenerations. It is part of Friedland’s ongoing research on the relationship between the microbiota and age-related brain disorders, which involves collaborations with researchers in Ireland and Japan.
Source: Betty Coffman – University of Louisville
Image Source: NeuroscienceNews.com image is credited to the researchers/Scientific Reports.
Original Research: Full open access research for “Exposure to the Functional Bacterial Amyloid Protein Curli Enhances Alpha-Synuclein Aggregation in Aged Fischer 344 Rats and Caenorhabditis elegans” by Shu G. Chen, Vilius Stribinskis, Madhavi J. Rane, Donald R. Demuth, Evelyne Gozal, Andrew M. Roberts, Rekha Jagadapillai, Ruolan Liu, Kyonghwan Choe, Bhooma Shivakumar, Francheska Son, Shunying Jin, Richard Kerber, Anthony Adame, Eliezer Masliah and Robert P. Friedland in Scientific Reports. Published online October 6 2016 doi:10.1038/srep34477
[cbtabs][cbtab title=”MLA”]University of Louisville “The Role Gut Bacteria Plays in Neurodegenerative Diseases.” NeuroscienceNews. NeuroscienceNews, 6 October 2016.
<https://neurosciencenews.com/gut-microbiome-neurodegenerative diseases-5221/>.[/cbtab][cbtab title=”APA”]University of Louisville (2016, October 6). The Role Gut Bacteria Plays in Neurodegenerative Diseases. NeuroscienceNew. Retrieved October 6, 2016 from https://neurosciencenews.com/gut-microbiome-neurodegenerative diseases-5221/[/cbtab][cbtab title=”Chicago”]University of Louisville “The Role Gut Bacteria Plays in Neurodegenerative Diseases.” https://neurosciencenews.com/gut-microbiome-neurodegenerative diseases-5221/ (accessed October 6, 2016).[/cbtab][/cbtabs]
Abstract
Exposure to the Functional Bacterial Amyloid Protein Curli Enhances Alpha-Synuclein Aggregation in Aged Fischer 344 Rats and Caenorhabditis elegans
Misfolded alpha-synuclein (AS) and other neurodegenerative disorder proteins display prion-like transmission of protein aggregation. Factors responsible for the initiation of AS aggregation are unknown. To evaluate the role of amyloid proteins made by the microbiota we exposed aged rats and transgenic C. elegans to E. coli producing the extracellular bacterial amyloid protein curli. Rats exposed to curli-producing bacteria displayed increased neuronal AS deposition in both gut and brain and enhanced microgliosis and astrogliosis compared to rats exposed to either mutant bacteria unable to synthesize curli, or to vehicle alone. Animals exposed to curli producing bacteria also had more expression of TLR2, IL-6 and TNF in the brain than the other two groups. There were no differences among the rat groups in survival, body weight, inflammation in the mouth, retina, kidneys or gut epithelia, and circulating cytokine levels. AS-expressing C. elegans fed on curli-producing bacteria also had enhanced AS aggregation. These results suggest that bacterial amyloid functions as a trigger to initiate AS aggregation through cross-seeding and also primes responses of the innate immune system.
“Exposure to the Functional Bacterial Amyloid Protein Curli Enhances Alpha-Synuclein Aggregation in Aged Fischer 344 Rats and Caenorhabditis elegans” by Shu G. Chen, Vilius Stribinskis, Madhavi J. Rane, Donald R. Demuth, Evelyne Gozal, Andrew M. Roberts, Rekha Jagadapillai, Ruolan Liu, Kyonghwan Choe, Bhooma Shivakumar, Francheska Son, Shunying Jin, Richard Kerber, Anthony Adame, Eliezer Masliah and Robert P. Friedland in Scientific Reports. Published online October 6 2016 doi:10.1038/srep34477






