Summary: Researchers report transplanting altered neural stem cells into the brains of mice genetically engineered to exhibit symptoms of ALS, delayed the progression of the disease and extended lifespan.
Source: Cedars Sinai Medical Center.
Investigators at Cedars-Sinai are exploring a new way to treat amyotrophic lateral sclerosis (ALS) by transplanting specially engineered neural cells into the brain. Their new study shows the transplanted cells delayed disease progression and extended survival in animal models.
ALS, also known as Lou Gehrig’s disease, is a neurological disorder that causes progressive paralysis and ultimately death. Although drugs and mechanical devices can help alleviate symptoms such as muscle spasms, there is no effective treatment, and most patients die within five years. More than 12,000 people in the U.S. have ALS, according to the National Institutes of Health.
“If we are able in the future to reproduce our research results in humans, we could improve both the quality and length of life for patients diagnosed with this devastating disease,” said Gretchen Thomsen, PhD, assistant professor of Biomedical Sciences and a research scientist at the Cedars-Sinai Board of Governors Regenerative Medicine Institute. Thomsen was the first author of the study, published today in the journal Stem Cells.
For the study, investigators genetically reprogrammed neural progenitor cells to secrete a special protein known as GDNF and then transplanted the cells into the brain cortices of animal models of ALS. GDNF helps sustain glial cells, which support the body’s motor neurons. In ALS patients, glial cells lack certain proteins and become sick, and the motor neurons gradually die off, causing paralysis.
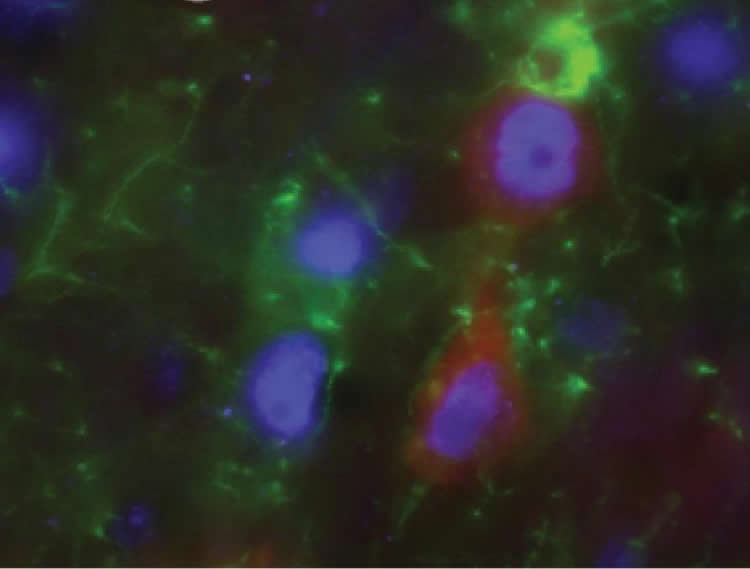
Once inside the cortex, the transplanted cells in the study matured into glial cells and released GDNF into the brain–a major scientific achievement in itself. Laboratory rats that received the transplants lived 8 percent longer and were free of paralysis 10 percent longer than were untreated animals. Motor neurons in the spine, which control muscle movement, also survived longer in the experimental group.
While the research results showed promise, more preclinical studies are needed to determine which treatment levels may be adequate and safe, Thomsen said. Cedars-Sinai investigators currently are performing these studies as preliminary steps to creating proposals for clinical trials.
A Cedars-Sinai team already is conducting a clinical trial that uses similarly reprogrammed, GDNF-producing progenitor cells and transplants them into the spinal cords of ALS patients. The trial, announced in 2016, is ongoing. Other initiatives at Cedars-Sinai that seek to understand ALS and develop effective treatments for it include a study published in March in Stem Cell Reports that revealed the brain’s blood vessels can activate genes known to trigger spinal motor neurons to grow, and a 2016 study published in the journal Science that found immune cells in the brain play a direct role in the development of ALS.
“Cedars-Sinai is committed to pursuing groundbreaking research that aims to one day eradicate ALS and the profound human suffering of this disease,” said Shlomo Melmed, MB, ChB, executive vice president, Academic Affairs, and dean of the medical faculty at Cedars-Sinai. “Today’s study significantly advances that drive.”
Besides Thomsen, the study team included senior author Clive Svendsen, PhD, professor of Biomedical Sciences and Medicine and director of the Cedars-Sinai Board of Governors Regenerative Medicine Institute–and co-authors from the Institute, the Biobehavioral Research Core and the Department of Biomedical Sciences at Cedars-Sinai, as well as from the University of California, San Francisco.
Funding: Research reported in this news release was supported by ALS Finding a Cure®, a program of The Leandro P. Rizzuto Foundation; the ALS Association; and the U.S. Department of Defense under award number W81XWH-14-1-0189.
Source: Jane Engle – Cedars Sinai Medical Center
Publisher: Organized by NeuroscienceNews.com.
Image Source: NeuroscienceNews.com image is credited to Cedars-Sinai Board of Governors Regenerative Medicine Institute.
Original Research: Open access research for “Transplantation of Neural Progenitor Cells Expressing Glial Cell Line‐Derived Neurotrophic Factor into the Motor Cortex as a Strategy to Treat Amyotrophic Lateral Sclerosis” by Gretchen M. Thomsen, Pablo Avalos, Annie A. Ma, Mor Alkaslasi, Noell Cho, Livia Wyss, Jean‐Philippe Vit, Marlesa Godoy, Patrick Suezaki, Oksana Shelest, Krystof S. Bankiewicz, and Clive N. Svendsen in Stem Cells. Published April 15 2018.
doi:10.1002/stem.2825
[cbtabs][cbtab title=”MLA”]Cedars Sinai Medical Center “ALS Treatment Delays Disease and Extends Life: Mouse Study.” NeuroscienceNews. NeuroscienceNews, 18 April 2018.
<https://neurosciencenews.com/als-life-extended-8832/>.[/cbtab][cbtab title=”APA”]Cedars Sinai Medical Center (2018, April 18). ALS Treatment Delays Disease and Extends Life: Mouse Study. NeuroscienceNews. Retrieved April 18, 2018 from https://neurosciencenews.com/als-life-extended-8832/[/cbtab][cbtab title=”Chicago”]Cedars Sinai Medical Center “ALS Treatment Delays Disease and Extends Life: Mouse Study.” https://neurosciencenews.com/als-life-extended-8832/ (accessed April 18, 2018).[/cbtab][/cbtabs]
Abstract
Transplantation of Neural Progenitor Cells Expressing Glial Cell Line‐Derived Neurotrophic Factor into the Motor Cortex as a Strategy to Treat Amyotrophic Lateral Sclerosis
Early dysfunction of cortical motor neurons may underlie the initiation of amyotrophic lateral sclerosis (ALS). As such, the cortex represents a critical area of ALS research and a promising therapeutic target. In the current study, human cortical‐derived neural progenitor cells engineered to secrete glial cell line‐derived neurotrophic factor (GDNF) were transplanted into the SOD1G93A ALS rat cortex, where they migrated, matured into astrocytes, and released GDNF. This protected motor neurons, delayed disease pathology and extended survival of the animals. These same cells injected into the cortex of cynomolgus macaques survived and showed robust GDNF expression without adverse effects. Together this data suggests that introducing cortical astrocytes releasing GDNF represents a novel promising approach to treating ALS.






