Summary: A new study in mice reveals a possible link between the use of SSRI antidepressants during pregnancy and an increased risk of autism-like symptoms in offspring. Offspring exposed to fluoxetine (Prozac) in utero were more likely to exhibit impaired neurotransmission caused by an overactive serotonin receptor in the prefrontal cortex, an area of the brain implicated in modulating social behaviors. However, treating the mice with a compound that blocks the receptor alleviated the behavioral problems and improved working memory.
Source: Duke-NUS Medical School
An international team led by Duke-NUS Medical School has found a potential link between autistic-like behaviour in adult mice and exposure to a common antidepressant in the womb. They also identified a treatment that helped improve memory loss and social interactions, according to the new study published in the journal Molecular Brain.
Antidepressants are commonly prescribed for treating major depression and post-traumatic stress disorder, including in pregnant women. One of the most commonly prescribed antidepressants is fluoxetine, a serotonin reuptake inhibitor. Fluoxetine can cross the placenta and is also detected in breast milk. Little is known about its safety during pregnancy, and not enough studies have been conducted on its long-term effects on offspring.
“Many human association studies have been conducted to investigate connections between antidepressant exposure during pregnancy and children with autism and attention deficit disorder (ADHD). But they have not been able to pinpoint a causal relationship,” stated Associate Professor Hyunsoo Shawn Je, from Duke-NUS’ Neuroscience and Behavioural Disorders (NBD) Programme, a senior and corresponding author of the study.
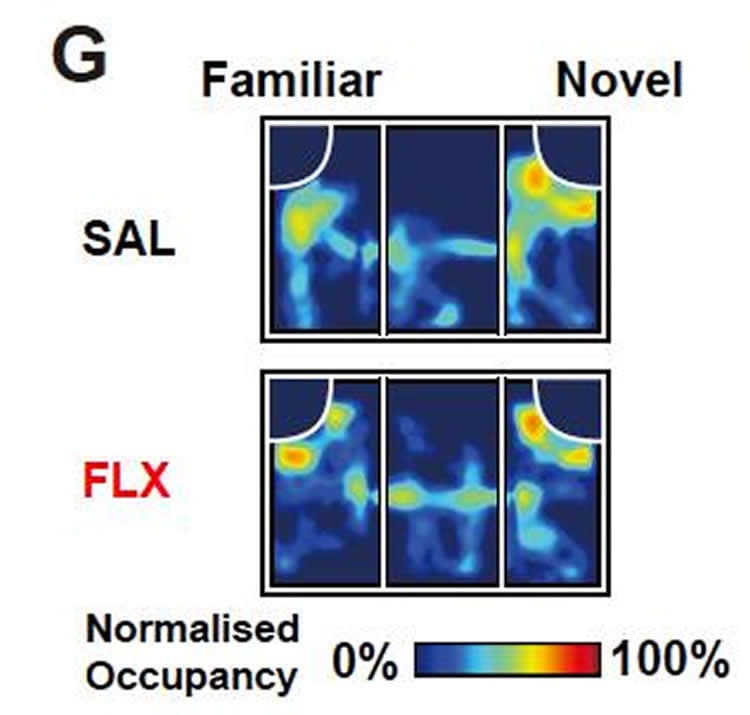
The team from Duke-NUS and their collaborators in South Korea and Singapore investigated adult mice born to mothers treated with fluoxetine (sold under the brand names Prozac and Sarafem) over a 15-day time period that corresponds to the second trimester in humans, in comparison with those born to mothers given normal saline as controls. They found key differences in behaviour. For example, the unexposed mice normally explored all three arms of a Y-shaped maze over a ten-minute time period and, over the courses of multiple arm entries, mice usually enter a less recently visited arm, while the fluoxetine-exposed ones were less inclined to explore unvisited arm.
In a second experiment, the mice were introduced to two juvenile mice, one after the other. When the second new mouse was introduced, mice that were not exposed to fluoxetine were more likely to only sniff the newly introduced mouse, recognizing that they had already met the first mouse. But the fluoxetine-exposed group sniffed both mice, indicating that they had impaired social novelty recognition.
The team then examined nerve signal transmission in the prefrontal cortex, a part of the brain involved in moderating social behaviour. They found impaired transmission caused by an overactive serotonin receptor. Treating fluoxetine-exposed mice with a compound that blocks the receptor alleviated their behavioural problems and improved their working memory.
The team next wants to examine autistic children born to mothers treated with antidepressants using positron emission tomography (PET) scans, an imaging technique used to observe metabolic processes in the body. If they also show enhanced serotonin receptor activity in the same area of the brain, the team plans to test whether FDA-approved serotonin receptor blockers can normalize their behaviours.
“The consensus among experts is that the rise in the number of people diagnosed with autism around the world is likely due to more awareness and testing rather than an increase in the prevalence of autism,” noted Professor Patrick Casey, Senior Vice Dean for Research at Duke-NUS. “This collaborative study by our researchers offers a compelling case for a link between autism and antidepressant exposure in the womb in an animal model, and a possible mechanism that could potentially be exploited for future therapies.”
Source:
Duke-NUS Medical School
Media Contacts:
Federico Graciano – Duke-NUS Medical School
Image Source:
The image is credited to Duke-NUS Medical School.
Original Research: Open access
“Prenatal selective serotonin reuptake inhibitor (SSRI) exposure induces working memory and social recognition deficits by disrupting inhibitory synaptic networks in male mice”. Weonjin Yu, Yi-Chun Yen, Young-Hwan Lee, Shawn Tan, Yixin Xiao, Hidayat Lokman, Audrey Khoo Tze Ting, Hasini Ganegala, Taejoon Kwon, Won-Kyung Ho and H. Shawn Je. Molecular Brain. doi:10.1186/s13041-019-0452-5
Abstract
Prenatal selective serotonin reuptake inhibitor (SSRI) exposure induces working memory and social recognition deficits by disrupting inhibitory synaptic networks in male mice
Selective serotonin reuptake inhibitors (SSRIs) are commonly prescribed antidepressant drugs in pregnant women. Infants born following prenatal exposure to SSRIs have a higher risk for behavioral abnormalities, however, the underlying mechanisms remains unknown. Therefore, we examined the effects of prenatal fluoxetine, the most commonly prescribed SSRI, in mice. Intriguingly, chronic in utero fluoxetine treatment impaired working memory and social novelty recognition in adult males. In the medial prefrontal cortex (mPFC), a key region regulating these behaviors, we found augmented spontaneous inhibitory synaptic transmission onto the layer 5 pyramidal neurons. Fast-spiking interneurons in mPFC exhibited enhanced intrinsic excitability and serotonin-induced excitability due to upregulated serotonin (5-HT) 2A receptor (5-HT2AR) signaling. More importantly, the behavioral deficits in prenatal fluoxetine treated mice were reversed by the application of a 5-HT2AR antagonist. Taken together, our findings suggest that alterations in inhibitory neuronal modulation are responsible for the behavioral alterations following prenatal exposure to SSRIs.






