Summary: Researchers use gene editing to generate aggressive glioblastoma multiforme brain tumors in the lab. The model, researchers say, could be used to track the progression of tumors and develop personalized therapies for patients.
Source: Salk Institute.
Glioblastoma multiforme (GBM) is an incredibly deadly brain cancer and presents a serious black box challenge. It’s virtually impossible to observe how these tumors operate in their natural environment and animal models don’t always provide good answers.
But now, Salk Institute researchers have taken an important step towards meeting that challenge. By editing two genes in just a few cells in human cerebral organoids, scientists in the Verma lab generated aggressive GBM tumors. This new model could be used to study tumor progression, investigate new drugs or even personalize treatments for patients. The study was published in the journal Cell Reports on April 24, 2018.
One of the problems plaguing clinical trials is, quite often, drugs that work in animals do not work in people. Researchers have tried to overcome this by using xenografts, in which patient tumor tissue is implanted in animal models, but this approach has its own issues. Sometimes, there isn’t enough human tumor tissue to study and, over time, the tumors adapt to their new home.
“As tumors grow in mice, the environment changes the tumor’s features,” says Junko Ogawa, a Salk senior research associate and first author on the paper. “We don’t know if it’s similar to the patient’s original cancer.”
The solution could be human cerebral organoids, which contain neurons and other brain cells. The Salk lab has been using stem cells to generate these small (around 4 mm) 3D structures in a dish for some time and wanted to investigate how they could be applied to study GBM.
They used the CRISPR-Cas9 tool to edit two genes closely associated with cancer, HRas and p53, in a few cells in an organoid. HRas is a cancer oncogene that drives rampant cell growth, while p53 is a tumor suppressor. In other words, they took their foot off the brake and stomped on the gas.
These organoids turned into tumor-like structures in the dish–they grew aggressively and had several biomarkers associated with GBM. Eventually, they took over the organoids, supplanting the original cells with tumor tissue. In addition, they could be serially transplanted into animal models, where they were also quite aggressive.
This approach offers a number of advantages. Editing p53 and HRas in just a few cells better replicates how GBMs actually develop in people–they don’t start as thousands of cells at once (like a xenograft) but rather as one or two aberrant cells.
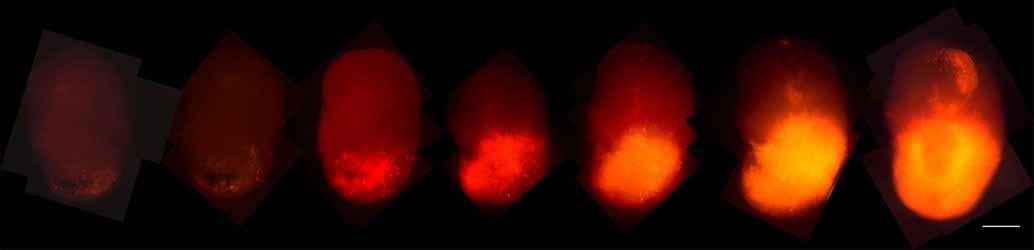
The team added a fluorescent red marker, called tdTomato, to the oncogenic HRas. As those cells took over the organoids, the researchers could track their progression. In addition, when the organoid tumors were transplanted into the brains of mice, they grew rapidly and resembled tumors taken from patients, offering easier access to samples.
“You can phenocopy the properties of the tumors in a mouse,” says Ogawa, “and now we can give them drugs to see if they are effective. We can also test the tumor’s ability to invade normal brain tissue.”
These organoids could also host human tumor samples and some GBM cell lines. This model could be used to personalize care. Researchers and clinicians could transplant the cancer cells from patients to make organoid models. As a result, they could study how a tumor responds to treatment in cells that match the patient’s genome. While the organoids lack endothelial cells and an immune system (which would give them more complexity and help them better replicate actual brain tissue), this model could be quite useful in studying a variety of brain metastatic cancers, not just GBM.
Funding: This work was funded by the National Institutes of Health (R01CA095613, P30 CA014195-38, P30 014195, P30 014195 and P30 014195), the H.N. and Frances C. Berger Foundation, the Leona M. and Harry B. Helmsley Charitable Trust (grant 2017-PG-MED001), the Glenn Center for Aging Research and the Chapman Foundation.
Source: Salk Institute
Publisher: Organized by NeuroscienceNews.com.
Image Source: NeuroscienceNews.com image is credited to Salk Institute.
Original Research: Open access research for “Glioblastoma Model Using Human Cerebral Organoids” by Junko Ogawa, Gerald M. Pao, Maxim N. Shokhirev, Inder M. Verma in Cell Reports. Published April 24 2018.
doi:10.1016/j.celrep.2018.03.105
[cbtabs][cbtab title=”MLA”]Salk Institute “Organoids Reveal how Deadly Brain Cancers Grow.” NeuroscienceNews. NeuroscienceNews, 25 April 2018.
<https://neurosciencenews.com/organoids-brain-cancer-8883/>.[/cbtab][cbtab title=”APA”]Salk Institute (2018, April 25). Organoids Reveal how Deadly Brain Cancers Grow. NeuroscienceNews. Retrieved April 25, 2018 from https://neurosciencenews.com/organoids-brain-cancer-8883/[/cbtab][cbtab title=”Chicago”]Salk Institute “Organoids Reveal how Deadly Brain Cancers Grow.” https://neurosciencenews.com/organoids-brain-cancer-8883/ (accessed April 25, 2018).[/cbtab][/cbtabs]
Abstract
Glioblastoma Model Using Human Cerebral Organoids
Highlights
•Human cerebral organoids can be used as a model for tumor formation
•Oncogene manipulation by CRISPR/Cas9 initiates tumorigenesis in cerebral organoids
•Time-lapse microscopic imaging allows observation of tumor development in organoids
•Tumor cells derived from organoids display an invasive phenotype in xenografted mice
Summary
We have developed a cancer model of gliomas in human cerebral organoids that allows direct observation of tumor initiation as well as continuous microscopic observations. We used CRISPR/Cas9 technology to target an HRasG12V-IRES-tdTomato construct by homologous recombination into the TP53 locus. Results show that transformed cells rapidly become invasive and destroy surrounding organoid structures, overwhelming the entire organoid. Tumor cells in the organoids can be orthotopically xenografted into immunodeficient NOD/SCID IL2RG−/− animals, exhibiting an invasive phenotype. Organoid-generated putative tumor cells show gene expression profiles consistent with mesenchymal subtype human glioblastoma. We further demonstrate that human-organoid-derived tumor cell lines or primary human-patient-derived glioblastoma cell lines can be transplanted into human cerebral organoids to establish invasive tumor-like structures. Our results show potential for the use of organoids as a platform to test human cancer phenotypes that recapitulate key aspects of malignancy.