Summary: Severed axonal segments signal to Schwann cells to begin actin sphere formation and axon disintegration. If the process is disrupted, axon disintegration is slowed and axon fragments impair nerve regeneration.
Source: Johannes Gutenberg University Mainz
Damaged peripheral nerves can regenerate after an injury, for example, following a forearm fracture. Axons, the long projections of neurons that transmit stimuli or signals to other cells, are affected in the case of injury and need to regrow to recover their function. The research team led by Prof. Claire Jacob at Johannes Gutenberg University Mainz (JGU) and at the Swiss University of Fribourg investigated the details of this repair process and have demonstrated that the same mechanism could be activated in cells of the central nervous system – after a spinal cord injury, for instance. Their results have been published in the renowned journal Cell Reports.
“An injury in the peripheral nervous system quickly triggers the activation of a fascinating repair process that allows the injured nerve to regenerate and regain its function. There is no such repair process in the central nervous system, thus injuries often lead to permanent damage such as paraplegia,” explains Claire Jacob, Head of Cellular Neurobiology at JGU. Strategies to improve axon regeneration in the central nervous system must, therefore, be developed to enable healing.
Myelin-forming cells are key to the axon regeneration process. Many axons are ensheathed by myelin, which serves as a protective layer while also enabling fast and efficient signal transmission. “Myelin is extremely important for the function of the entire nervous system, however, it also hinders the repair process in case of an injury,” adds Claire Jacob. Myelin is produced by Schwann cells in the peripheral nervous system and by oligodendrocytes in the central nervous system; this difference has a major impact on axon regeneration because Schwann cells and oligodendrocytes respond very differently to axonal injury.
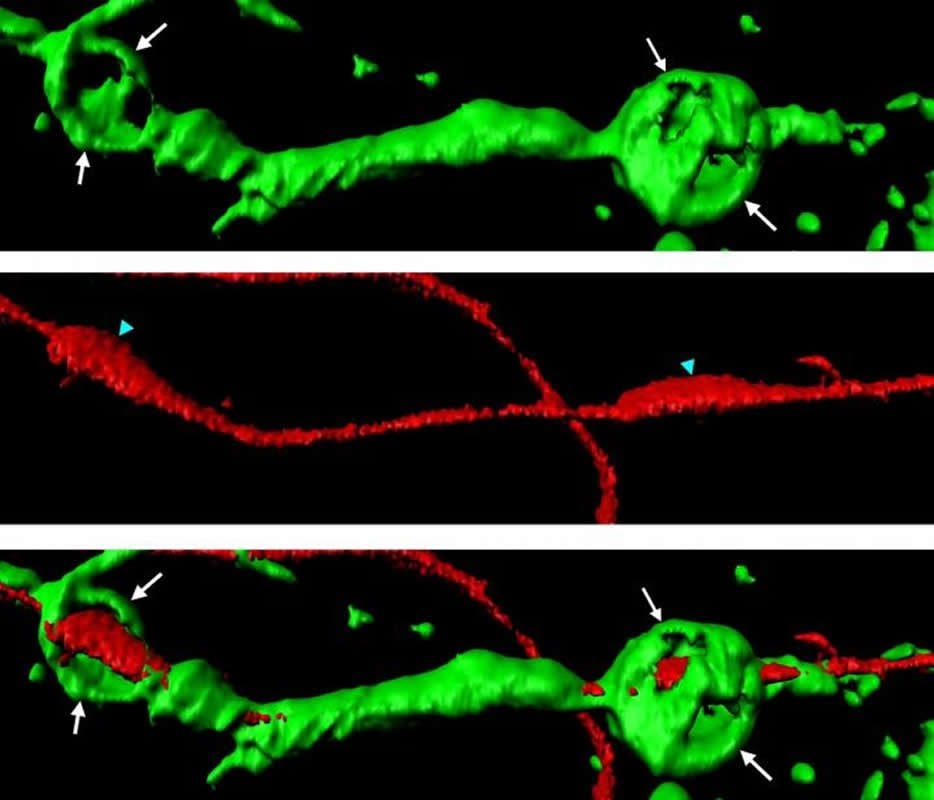
Schwann cells can do everything – they break down myelin and damaged axons
When axons of the peripheral nervous system are injured, Schwann cells rapidly induce the disintegration of the cut-out axonal segments into small fragments, which can then be digested by Schwann cells themselves or later by macrophages. This elimination of axonal debris is one of the first and critical steps of the repair process. “Schwann cells can do everything. We discovered that they not only digest myelin following injury, but they also induce the disintegration of the long axon segments that are separated from their cell bodies due to the injury,” points out Claire Jacob. In order to do that, Schwann cells form small spheres made of a protein called actin; these actin spheres exert pressure on the isolated axon segments until their disintegration into small pieces. This targeted degradation of cell debris is essential to enable the healthy part of the axon that remained attached to the neuron cell body to grow back, connect to its former target and thereby regain full functionality.
Severed axons transmit signals to Schwann cells
Of particular interest, the Jacob team found that severed axonal segments send a signal to Schwann cells that prompts them to start the actin sphere formation and axon disintegration process, an impressive and precisely coordinated form of interaction between the two cell types. If this mechanism is disrupted, axonal disintegration is slowed down and axonal fragments impair the regeneration of the affected nerve.
Manipulated oligodendrocytes can also generate actin structures
Claire Jacob’s team went on to study the central nervous system and the behavior of oligodendrocytes. “After an injury, oligodendrocytes either die or remain apparently unresponsive,” says Claire Jacob. Oligodendrocytes are not (normally) able, like Schwann cells, to form actin spheres and thus break down axon segments. One reason for this is that, unlike Schwann cells, they do not express VEGFR1, the receptor that triggers the production of actin spheres in Schwann cells. In the next step, the research team induced the expression of VEGFR1 in oligodendrocytes. This allowed oligodendrocytes to produce actin structures and disintegrate severed axonal fragments; this is an essential step to promote the regeneration of neurons in the central nervous system.
The team is currently working at identifying the molecular processes that trigger the removal of myelin at the site of injury in the central nervous system. In addition to the disposal of axonal debris, myelin removal is a second prerequisite necessary for the complete regeneration of neurons. “We have discovered a pathway that accelerates myelin degradation in the peripheral nervous system and are now trying to determine whether this can also trigger myelin removal in the central nervous system,” adds Claire Jacob, describing the results of on-going research in her lab.
Claire Jacob is the head of the Cellular Neurobiology Group at Johannes Gutenberg University Mainz since October 2018. The article, published in Cell Reports, includes findings from the research groups at the Universities of Fribourg (Switzerland) and Mainz. In September 2018, Claire Jacob was awarded the prestigious IRP Schellenberg Research Prize.
Source:
Johannes Gutenberg University Mainz
Media Contacts:
Dr. Claire Jacob – Johannes Gutenberg University Mainz
Image Source:
The image is credited to Adrien Vaquie.
Original Research: Open access
“Injured Axons Instruct Schwann Cells to Build Constricting Actin Spheres to Accelerate Axonal Disintegration”. Claire Jacob et al.
Cell Reports. doi:10.1016/j.celrep.2019.05.060
Abstract
Injured Axons Instruct Schwann Cells to Build Constricting Actin Spheres to Accelerate Axonal Disintegration
Highlights
• Injured axons upregulate PlGF, which activates VEGFR1 in Schwann cells
• VEGFR1 activates the formation of constricting actin spheres in Schwann cells
• Constricting actin spheres accelerate the disintegration of injured axons
• VEGFR1 expression in oligodendrocytes accelerates injured axon disintegration
Summary
After a peripheral nerve lesion, distal ends of injured axons disintegrate into small fragments that are subsequently cleared by Schwann cells and later by macrophages. Axonal debris clearing is an early step of the repair process that facilitates regeneration. We show here that Schwann cells promote distal cut axon disintegration for timely clearing. By combining cell-based and in vivo models of nerve lesion with mouse genetics, we show that this mechanism is induced by distal cut axons, which signal to Schwann cells through PlGF mediating the activation and upregulation of VEGFR1 in Schwann cells. In turn, VEGFR1 activates Pak1, leading to the formation of constricting actomyosin spheres along unfragmented distal cut axons to mediate their disintegration. Interestingly, oligodendrocytes can acquire a similar behavior as Schwann cells by enforced expression of VEGFR1. These results thus identify controllable molecular cues of a neuron-glia crosstalk essential for timely clearing of damaged axons.






