Heidelberg researchers discover central mechanism and identify key neuroprotective molecule.
Activated neurons produce a protein that protects against nerve cell death. Prof. Dr. Hilmar Bading and his group at Heidelberg University’s Interdisciplinary Center for Neuroscience have found out how this effect comes about and defined a crucial player. “We already knew that brain activity promotes neuroprotection,” Prof. Bading says. “Now we have discovered a central mechanism for this process and a key molecule produced by the body to develop a neuroprotective shield.” These results have been published in “Cell Reports”.
When nerve cells die, e.g. as a result of a stroke, Alzheimer’s disease or through age-related processes, the result may be considerable impairments of memory. Earlier studies led by Prof. Bading have shown that brain activity counteracts the death of nerve cells. The NMDA receptor plays a major role at the molecular level. This type of receptor is a molecule set in motion by biochemical neurotransmitters. Due to neuronal activity, the NMDA receptor causes calcium to enter the cell. The calcium signal is spreading within the cell, invades the cell nucleus and switches on a genetic protection programme. Prof. Bading’s group identified this nuclear calcium-induced gene programme a few years ago. “However, it was not clear to us how it leads to a protective shield,” Hilmar Bading explains.
The scientists have now discovered the explanation for this – again by studying NMDA receptors. If these receptors are not located at the neuronal junctions, i.e. the synapses, they do not contribute to the protection of cells. On the contrary, they severely damage nerve cells and cause them to die. “Life and death are only a few thousandths of a millimetre away from one another. Outside the synapse the NMDA receptor is no longer Dr. Jekyll, it becomes Mr. Hyde,” Hilmar Bading comments. The current research results show that toxic extrasynaptic NMDA receptors are suppressed through brain activity. The Heidelberg research team has identified activin A as the protein activating this process.
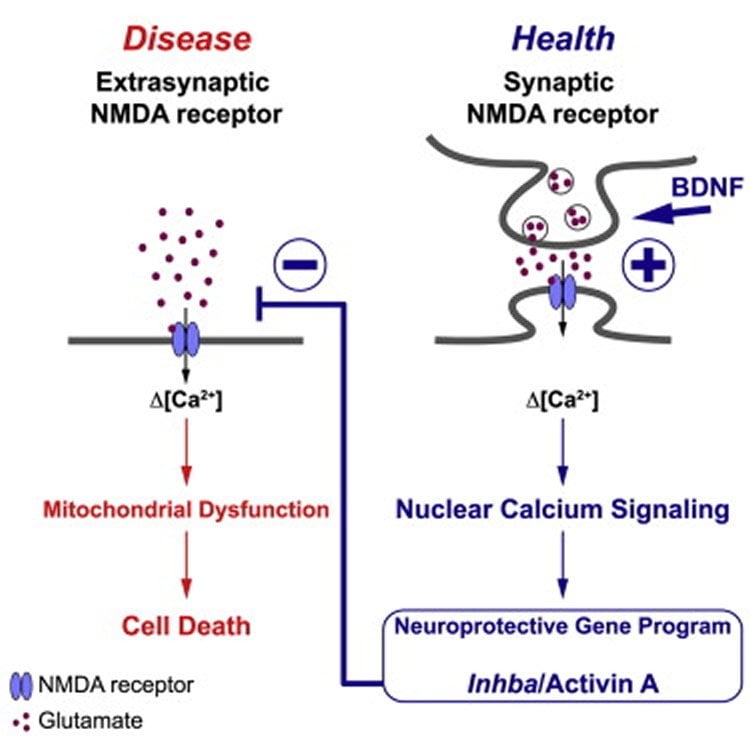
Activin A plays an important role e.g. in the menstruation cycle and in healing wounds. It is produced in the nervous system thanks to neuronal activity. This leads to a reduction in extrasynaptic NMDA receptors and builds up a protective shield, according to Prof. Bading. Activin A also mediates the well-known protective properties of brain-derived neurotrophic factor (BDNF), a signalling molecule that protects existing neurons and synapses, and helps developing new ones. “Activin A can therefore be regarded as a crucial activator of a common neuroprotective mechanism in the brain.”
The discoveries made by the Heidelberg neurobiologists open up new prospects for treating degenerative diseases of the nervous system. In their study they showed that activin A in mice was able to significantly reduce brain damage after a stroke. “Our research results also indicate that activin A may possibly be used to treat Alzheimer’s disease or Huntington’s disease. The characteristic degeneration of nerve cells associated with these two diseases seems to be due to an increased activity of toxic extrasynaptic NMDA receptors,” says Prof. Bading. “In everyday terms the new findings mean: An active brain produces activin A thereby protecting itself from neurodegeneration.”
Source: Heidelberg University
Image Credit: The image is credited to Bading et al./Cell Reports and is licensed CC BY-NC-ND 4.0
Original Research: Full open access research for “BDNF Reduces Toxic Extrasynaptic NMDA Receptor Signaling via Synaptic NMDA Receptors and Nuclear-Calcium-Induced Transcription of inhba/Activin A” by David Lau, C. Peter Bengtson, Bettina Buchthal, and Hilmar Bading in Cell Reports. Published online August 13 2015 doi:10.1016/j.celrep.2015.07.038
Abstract
BDNF Reduces Toxic Extrasynaptic NMDA Receptor Signaling via Synaptic NMDA Receptors and Nuclear-Calcium-Induced Transcription of inhba/Activin A
Highlights
•BDNF-induced neuroprotection requires synaptic NMDA receptors and nuclear calcium
•BDNF-nuclear calcium signaling induces transcription of inhba/activin A
•Activin A reduces toxic extrasynaptic NMDA receptor signaling, shielding mitochondria
•Activin A protects against excitotoxic cell death in cultured neurons and in vivo
Summary
The health of neurons is critically dependent on the relative signaling intensities of survival-promoting synaptic and death-inducing extrasynaptic NMDA receptors. Here, we show that BDNF is a regulator of this balance and promotes neuroprotection by reducing toxic NMDA receptor signaling. BDNF acts by initiating synaptic NMDA-receptor/nuclear-calcium-driven adaptogenomics, leading to increased expression of inhibin β-A (inhba). Inhibin β-A (its homodimer is known as activin A) in turn reduces neurotoxic extrasynaptic NMDA-receptor-mediated calcium influx, thereby shielding neurons against mitochondrial dysfunction, a major cause of excitotoxicity. Thus, BDNF induces acquired neuroprotection by enhancing synaptic activity and lowering extrasynaptic NMDA receptor death signaling through a nuclear calcium-inhibin β-A pathway. This process, which confers protection against ischemic brain damage in a mouse stroke model, may be compromised in Huntington’s disease, Alzheimer’s disease, or aging-related neurodegenerative conditions that are associated with reduced BDNF levels and/or enhanced extrasynaptic NMDA receptor signaling.
“BDNF Reduces Toxic Extrasynaptic NMDA Receptor Signaling via Synaptic NMDA Receptors and Nuclear-Calcium-Induced Transcription of inhba/Activin A” by David Lau, C. Peter Bengtson, Bettina Buchthal, and Hilmar Bading in Cell Reports. Published online August 13 2015 doi:10.1016/j.celrep.2015.07.038







