In the early stages of Alzheimer’s disease, patients are often unable to remember recent experiences. However, a new study from MIT suggests that those memories are still stored in the brain, they just can’t be easily accessed.
The MIT neuroscientists report in Nature that mice in the early stages of Alzheimer’s can form new memories just as well as normal mice but cannot recall them a few days later.
Furthermore, the researchers were able to artificially stimulate those memories using a technique known as optogenetics, suggesting that those memories can still be retrieved with a little help. Although optogenetics cannot currently be used in humans, the findings raise the possibility of developing future treatments that might reverse some of the memory loss seen in early-stage Alzheimer’s, the researchers say.
“The important point is, this a proof of concept. That is, even if a memory seems to be gone, it is still there. It’s a matter of how to retrieve it,” says Susumu Tonegawa, the Picower Professor of Biology and Neuroscience and director of the RIKEN-MIT Center for Neural Circuit Genetics at the Picower Institute for Learning and Memory.
Tonegawa is the senior author of the study, which appears in the March 16 online edition of Nature. Dheeraj Roy, an MIT graduate student, is the paper’s lead author.
Lost memories
In recent years, Tonegawa’s lab has identified cells in the brain’s hippocampus that store specific memories. The researchers have also shown that they can manipulate these memory traces, or engrams, to plant false memories, activate existing memories, or alter a memory’s emotional associations.
Last year, Tonegawa, Roy, and colleagues found that mice with retrograde amnesia, which follows traumatic injury or stress, had impaired memory recall but could still form new memories. That led the team to wonder whether this might also be true for the memory loss seen in the early stages of Alzheimer’s disease, which occurs before characteristic amyloid plaques appear in patients’ brains.
To investigate that possibility, the researchers studied two different strains of mice genetically engineered to develop Alzheimer’s symptoms, plus a group of healthy mice.
All of these mice, when exposed to a chamber where they received a foot shock, showed fear when placed in the same chamber an hour later. However, when placed in the chamber again several days later, only the normal mice still showed fear. The Alzheimer’s mice did not appear to remember the foot shock.
“Short-term memory seems to be normal, on the order of hours. But for long-term memory, these early Alzheimer’s mice seem to be impaired,” Roy says.
“An access problem”
The researchers then showed that while the mice cannot recall their experiences when prompted by natural cues, those memories are still there.
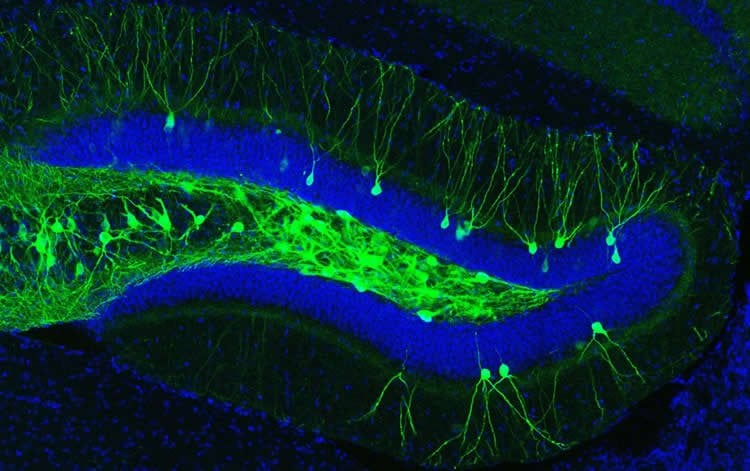
To demonstrate this, they first tagged the engram cells associated with the fearful experience with a light-sensitive protein called channelrhodopsin, using a technique they developed in 2012. Whenever these tagged engram cells are activated by light, normal mice recall the memory encoded by that group of cells. Likewise, when the researchers placed the Alzheimer’s mice in a chamber they had never seen before and shined light on the engram cells encoding the fearful experience, the mice immediately showed fear.
“Directly activating the cells that we believe are holding the memory gets them to retrieve it,” Roy says. “This suggests that it is indeed an access problem to the information, not that they’re unable to learn or store this memory.”
The researchers also showed that the engram cells of Alzheimer’s mice had fewer dendritic spines, which are small buds that allow neurons to receive incoming signals from other neurons.
Normally, when a new memory is generated, the engram cells corresponding to that memory grow new dendritic spines, but this did not happen in the Alzheimer’s mice. This suggests that the engram cells are not receiving sensory input from another part of the brain called the entorhinal cortex. The natural cue that should reactivate the memory — being in the chamber again — has no effect because the sensory information doesn’t get into the engram cells.

“If we want to recall a memory, the memory-holding cells have to be reactivated by the correct cue. If the spine density does not go up during learning process, then later, if you give a natural recall cue, it may not be able to reach the nucleus of the engram cells,” Tonegawa says.
Long-term connection
The researchers were also able to induce a longer-term reactivation of the “lost” memories by stimulating new connections between the entorhinal cortex and the hippocampus.
To achieve this, they used light to optogenetically stimulate entorhinal cortex cells that feed into the hippocampal engram cells encoding the fearful memory. After three hours of this treatment, the researchers waited a week and tested the mice again. This time, the mice could retrieve the memory on their own when placed in the original chamber, and they had many more dendritic spines on their engram cells.
However, this approach does not work if too large a section of the entorhinal cortex is stimulated, suggesting that any potential treatments for human patients would have to be very targeted. Optogenetics is very precise but too invasive to use in humans, and existing methods for deep brain stimulation — a form of electrical stimulation sometimes used to treat Parkinson’s and other diseases — affect too much of the brain.
“It’s possible that in the future some technology will be developed to activate or inactivate cells deep inside the brain, like the hippocampus or entorhinal cortex, with more precision,” Tonegawa says. “Basic research as conducted in this study provides information on cell populations to be targeted, which is critical for future treatments and technologies.”
Source: Anne Trafton – MIT
Image Source: The image of a woman’s head is credited to Jose-Luis Olivares/MIT. The engram cells image is credited to RIKEN.
Original Research: Abstract for “Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease” by Dheeraj S. Roy, Autumn Arons, Teryn I. Mitchell, Michele Pignatelli, Tomás J. Ryan and Susumu Tonegawa in Nature. Published online March 16 2016 doi:10.1038/nature17172
Abstract
Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by progressive memory decline and subsequent loss of broader cognitive functions1. Memory decline in the early stages of AD is mostly limited to episodic memory, for which the hippocampus has a crucial role2. However, it has been uncertain whether the observed amnesia in the early stages of AD is due to disrupted encoding and consolidation of episodic information, or an impairment in the retrieval of stored memory information. Here we show that in transgenic mouse models of early AD, direct optogenetic activation of hippocampal memory engram cells results in memory retrieval despite the fact that these mice are amnesic in long-term memory tests when natural recall cues are used, revealing a retrieval, rather than a storage impairment. Before amyloid plaque deposition, the amnesia in these mice is age-dependent3, 4, 5, which correlates with a progressive reduction in spine density of hippocampal dentate gyrus engram cells. We show that optogenetic induction of long-term potentiation at perforant path synapses of dentate gyrus engram cells restores both spine density and long-term memory. We also demonstrate that an ablation of dentate gyrus engram cells containing restored spine density prevents the rescue of long-term memory. Thus, selective rescue of spine density in engram cells may lead to an effective strategy for treating memory loss in the early stages of AD.
“Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease” by Dheeraj S. Roy, Autumn Arons, Teryn I. Mitchell, Michele Pignatelli, Tomás J. Ryan and Susumu Tonegawa in Nature. Published online March 16 2016 doi:10.1038/nature17172