Summary: Researchers report on how Schwann cells transform to play a more active role in neurogenesis following nerve injury.
Source: University of Wisconsin–Madison.
Researchers at the University of Wisconsin–Madison have found a switch that redirects helper cells in the peripheral nervous system into “repair” mode, a form that restores damaged axons.
Axons are long fibers on neurons that transmit nerve impulses. The peripheral nervous system, the signaling network outside the brain and spinal cord, has some ability to regenerate destroyed axons, but the repair is slow and often insufficient.
The new study suggests tactics that might trigger or accelerate this natural regrowth and assist recovery after physical injury, says John Svaren, a professor of comparative biosciences at the UW–Madison School of Veterinary Medicine. The finding may also apply to genetic abnormalities such as Charcot-Marie-Tooth disease or nerve damage from diabetes.
Svaren, senior author of a report published Aug. 30 in The Journal of Neuroscience, studied how Schwann cells, which hug axons in the peripheral nervous system, transform themselves to play a much more active and “intelligent” role after injury.
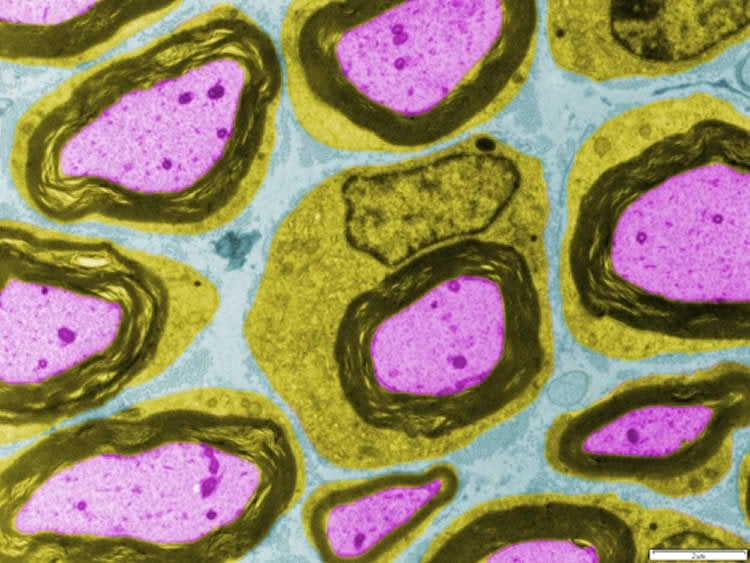
Schwann cells create the insulating myelin sheath that speeds transmission of nerve impulses. In the repair mode, Schwann cells form a fix-up crew that adds house cleaning and stimulation of nerve regrowth to the usual insulating job.
Svaren and his graduate student, Joseph Ma, compared the activation of genes in Schwann cells in mice with intact or cut axons. “We saw a set of latent genes becoming active, but only after injury,” says Svaren, “and these started a program that places the Schwann cells in a repair mode where they perform several jobs that the axon needs to regrow.”
In the repair mode, but not in the normal one, Schwann cells start cleaning house, helping to dissolve myelin, which is essential for proper functioning but ironically deters regeneration after injury. “If you invite Schwann cells to a party,” says Svaren, “they will clean up the bottles and wash your dishes before they leave the house.”
This cleanup must happen within days of the injury, says Svaren, who directs the cellular and molecular neuroscience core at the Waisman Center on the UW–Madison campus.
The Schwann cells also secrete signals that summon blood cells to aid the cleanup, and they map out a pathway for the axon to regrow. Finally, they return to the insulator role to grow a replacement myelin sheath on the regenerated axon.
Unexpectedly, the Schwann’s transition into the repair form did not entail a reversion to a more primitive form, but rather was based on a change in the regulation of its genes. “Almost every other nervous-system injury response, especially in the brain, is thought to require stem cells to repopulate the cells, but there are no stem cells here,” Svaren says. “The Schwann cells are reprogramming themselves to set up the injury-repair program. We are starting to see them as active players with dual roles in protecting and regenerating the axon, and we are exploring which factors determine the initiation and efficacy of the injury program.”
After the human genome was deciphered, epigenetics — the study of gene regulation — has moved to the forefront with the realization that genes don’t matter much until they are switched on, and that genetic switches are the fundamental reason why a skin cell doesn’t look like a nerve cell, and a nerve cells functions differently than a white blood cell.
In epigenetics, as elsewhere in biology, processes are often regulated through a balance between “stop” and “go” signals. In the Schwann cell transition, Svaren and Ma identified a system called PRC2 that usually silences the repair program. “This pathway amounts to an on-off switch that is normally off,” Svaren says, “and we want to know how to turn it on to initiate the repair process.”
The nature of the top-level gene-silencing system suggested drugs that might remove the silencing mark from the genes in question, and Svaren says he’s identified an enzyme that may “remove the brakes” and deliberately activate the repair program when needed in response to injury.
Even if the drug tests are promising, years of experiments will be necessary before the system can be tested in people. Furthermore, as Svaren acknowledges, “many factors determine how well an axon can regenerate. I am not saying this single pathway could lead to a cure-all, but we do hope it is an important factor.”
Svaren says it’s not clear how the current finding on peripheral nerves relates to damage to the brain and spinal cord, where a different type of cell cares for neurons. There are some similarities, however. In multiple sclerosis, for example, cleanup must precede the replacement of damaged myelin.
Ultimately, the study could open a new door on regeneration, even beyond one key sector of the nervous system. “We have thought of the Schwann cell as a static entity that was just there to make myelin, but they have this latent program, where they become the first responders and initiate many actions that are required for the axon to regenerate,” Svaren says.
Source: David Tenenbaum – University of Wisconsin–Madison
Image Source: This NeuroscienceNews.com image is credited to John Svaren.
Original Research: Abstract for “Epigenomic Regulation of Schwann Cell Reprogramming in Peripheral Nerve Injury” by Ki H. Ma, Holly A. Hung, and John Svaren in Journal of Neuroscience. Published online August 31 2016 doi:10.1523/JNEUROSCI.1370-16.2016
[cbtabs][cbtab title=”MLA”]University of Wisconsin–Madison . “Study Finds Key to Nerve Regeneration.” NeuroscienceNews. NeuroscienceNews, 14 September 2016.
<https://neurosciencenews.com/neurogenesis-axons-neuroscience-5043/>.[/cbtab][cbtab title=”APA”]University of Wisconsin–Madison . (2016, September 14). Study Finds Key to Nerve Regeneration. NeuroscienceNews. Retrieved September 14, 2016 from https://neurosciencenews.com/neurogenesis-axons-neuroscience-5043/[/cbtab][cbtab title=”Chicago”]University of Wisconsin–Madison . “Study Finds Key to Nerve Regeneration.” https://neurosciencenews.com/neurogenesis-axons-neuroscience-5043/ (accessed September 14, 2016).[/cbtab][/cbtabs]
Abstract
Epigenomic Regulation of Schwann Cell Reprogramming in Peripheral Nerve Injury
The rapid and dynamic transcriptional changes of Schwann cells in response to injury are critical to peripheral nerve repair, yet the epigenomic reprograming that leads to the induction of injury-activated genes has not been characterized. Polycomb Repressive Complex 2 (PRC2) catalyzes the trimethylation of lysine 27 of histone H3 (H3K27me3), which produces a transcriptionally repressive chromatin environment. We find that many promoters and/or gene bodies of injury-activated genes of mature rat nerves are occupied with H3K27me3. In contrast, the majority of distal enhancers that gain H3K27 acetylation after injury are not repressed by H3K27 methylation before injury, which is normally observed in developmentally poised enhancers. Injury induces demethylation of H3K27 in many genes, such as Sonic hedgehog (Shh), which is silenced throughout Schwann cell development before injury. In addition, experiments using a Schwann cell-specific mouse knock-out of the Eed subunit of PRC2 indicate that demethylation is a rate-limiting step in the activation of such genes. We also show that some transcription start sites of H3K27me3-repressed injury genes of uninjured nerves are bound with a mark of active promoters H3K4me3, for example, Shh and Gdnf, and the reduction of H3K27me3 results in increased trimethylation of H3K4. Our findings identify reversal of polycomb repression as a key step in gene activation after injury.
SIGNIFICANCE STATEMENT Peripheral nerve regeneration after injury is dependent upon implementation of a novel genetic program in Schwann cells that supports axonal survival and regeneration. Identifying means to enhance Schwann cell reprogramming after nerve injury could be used to foster effective remyelination in the treatment of demyelinating disorders and in identifying pathways involved in regenerative process of myelination. Although recent progress has identified transcriptional determinants of successful reprogramming of the Schwann cell transcriptome after nerve injury, our results have highlighted a novel epigenomic pathway in which reversal of the Polycomb pathway of repressive histone methylation is required for activation of a significant number of injury-induced genes.
“Epigenomic Regulation of Schwann Cell Reprogramming in Peripheral Nerve Injury” by Ki H. Ma, Holly A. Hung, and John Svaren in Journal of Neuroscience. Published online August 31 2016 doi:10.1523/JNEUROSCI.1370-16.2016