Summary: Researchers discover a control mechanism for neurons involved in hunger and eating disorders.
Source: Tufts University.
New study sheds light on control mechanisms for neurons involved in hunger, obesity and anorexia.
Neurons in the brain that control hunger are regulated by AMPK, a protein activated during fasting, report researchers from Tufts University School of Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School in Neuron on July 6, 2016.
High AMPK activity levels significantly increase the firing of “hunger” neurons known as AgRP neurons, leading to greater food intake, body weight and fat mass in mouse models. Blocking AMPK activity in mice led to reduced hunger and AgRP neuron firing, even after fasting. The research team found that AMPK alters the ability of AgRP neurons to form synapses, or new connections from other neurons. The study sheds light on the biological mechanisms that regulate feeding behavior, and serves as a potential model for the broad study of synapse formation.
“We know that AgRP is a master neuron group that controls hunger, and it is critical that we understand how these neurons are regulated since dysfunction may lead to obesity or anorexia,” said study author Dong Kong, PhD, assistant professor of neuroscience at Tufts University School of Medicine.
Located in the hypothalamus, AgRP (agouti-related peptide)-expressing neurons play an important role in feeding behavior. Experiments have shown that over-activation of these “hunger” neurons can cause mice to continue eating even when full. When AgRP neuron activity is blocked, mice cease eating. While AgRP neurons are known to be activated by fasting and the hormone ghrelin (produced by an empty stomach), and inhibited by the hormone leptin (produced by fat cells), less is known about their underlying biological mechanisms.
To investigate, Kong and his colleagues focused on AMPK, a metabolic sensor protein involved in energy regulation that can be found in most cells. AMPK activity has been shown to be triggered by fasting and ghrelin, mirroring AgRP neuron activation patterns. But its role in neurons was unclear. The researchers began by developing a suite of genetic tools that allowed them to assess AMPK activity in mice and manipulate its signaling in only AgRP neurons, leaving other cell types unaltered.
Necessary and sufficient
The team found that AMPK activity in AgRP neurons was more than double in fasted animals compared to animals in a sated state. They then engineered mice with continuously activated AMPK protein. This caused increased AgRP neuron firing and higher food intake, body weight and fat mass in both fasted mice and mice that had unlimited access to food. Blocking AMPK activity produced the opposite effect and decreased AgRP neuron activity.
In addition to altering the frequency and rate of firing of AgRP neurons, AMPK also affected the formation of dendritic spines–structures on neurons involved in neurotransmission, which receive synaptic inputs from other neurons. In a previous study, Kong and his colleagues found that dendritic spine density directly correlated with the effect of AgRP neurons on hunger. In the current study, the team confirmed that high levels of AMPK activity caused high AgRP dendritic spine density, while blocking AMPK activation halted spine formation.
“We previously found that fasting can drive feeding behavior with increased synapse formation and firing activity in AgRP neurons, and we have now shown that AMPK is both necessary and sufficient for this to occur,” said Kong, who serves as faculty in the Neuroscience and Cell, Molecular & Developmental Biology programs at the Sackler School of Graduate Biomedical Sciences at Tufts. “Even in a fed state, mice with continuously active AMPK have higher AgRP activity, eat more, and develop obesity faster.”
Kong and his colleagues further examined how AMPK affected dendritic spine formation by studying PAKs, a group of proteins involved in synaptic plasticity that AMPK is known to act on. The team found that specifically blocking PAKs inhibited AgRP neuron activity and dendritic spine formation in fasted mice–even in mice with AMPK continuously activated. Their findings suggest that PAKs are required for AMPK activation of AgRP neurons, and play an important role in AMPK-mediated synaptic plasticity.
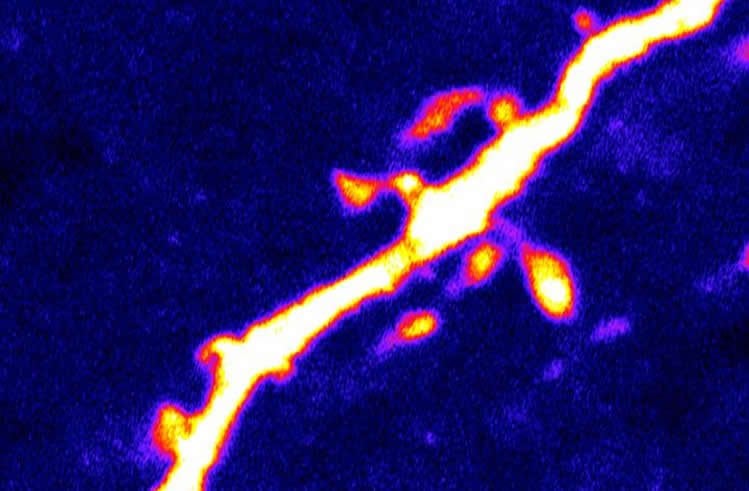
Kong cautions that AMPK and PAKs are currently not ideal therapeutic targets, due to their widespread presence in cells throughout the body. However, clarifying the biological mechanisms by which AgRP neurons function is necessary for future research efforts to identify more suitable targets, he says. Kong and his laboratory at Tufts are now further exploring the mechanisms by which AMPK, PAKs and AgRP neurons interact with each other, and with other signaling factors important for feeding such as leptin, ghrelin, insulin and glucose.
“Given the wide expression of AMPK and PAK proteins, as well as other engaged molecules, our findings could have potentially broader effects on the study of synapse formation,” Kong said. “While we do not provide a definitive target for drug development against obesity or eating disorders, we believe these findings clarify our understanding of this important energy-regulating pathway, and enable new approaches for future research and therapeutic studies.”
Kong carried out much of the work of this study while in the laboratories of co-corresponding authors Brad Lowell, professor of medicine at Beth Israel Deaconess Medical Center, and Bernardo Sabatini, professor of neurobiology at Harvard Medical School. Additional corresponding authors include Barbara Kahn, professor of medicine at Beth Israel Deaconess Medical Center. Additional contributing authors include Xinchi Yi and Yikun Guo, research assistants in Kong’s lab at Tufts, and Yossi Dagon, John N. Campbell, Zongfang Yang, Pratik Aryal and Kerry Wellenstein at Harvard Medical School.
Funding: The study was supported by awards from the National Institute of Diabetes and Digestive and Kidney Diseases (DK096010, DK089044, DK071051, DK075632, DK053477, DK094943, DK108797, DK098002, DK094943, DK108797) and National Institute of Neurological Disorders and Stroke (NS046579 and NS097922) at the National Institutes of Health, Boston Nutrition Obesity Research Center (DK046200), Boston Area Diabetes Endocrinology Research Center (DK57521), Charles Hood Foundation Grant and the American Heart Association.
Source: Kevin Jiang – Tufts University
Image Source: This NeuroscienceNews.com image is credited to Dong Kong.
Original Research: Abstract for “A Postsynaptic AMPK→p21-Activated Kinase Pathway Drives Fasting-Induced Synaptic Plasticity in AgRP Neurons” by Dong Kong, Yossi Dagon, John N. Campbell, Yikun Guo, Zongfang Yang, Xinchi Yi, Pratik Aryal, Kerry Wellenstein, Barbara B. Kahn, Bernardo L. Sabatini, and Bradford B. Lowell in Neuron. Published online June 16 2016 doi:10.1016/j.neuron.2016.05.025
[cbtabs][cbtab title=”MLA”]Tufts University. “Hunger Neurons Are Regulated by a Protein Activated During Fasting.” NeuroscienceNews. NeuroscienceNews, 6 July 2016.
<https://neurosciencenews.com/ampk-hunger-neurons-4624/>.[/cbtab][cbtab title=”APA”]Tufts University. (2016, July 6). Hunger Neurons Are Regulated by a Protein Activated During Fasting. NeuroscienceNew. Retrieved July 6, 2016 from https://neurosciencenews.com/ampk-hunger-neurons-4624/[/cbtab][cbtab title=”Chicago”]Tufts University. “Hunger Neurons Are Regulated by a Protein Activated During Fasting.” https://neurosciencenews.com/ampk-hunger-neurons-4624/ (accessed July 6, 2016).[/cbtab][/cbtabs]
Abstract
A Postsynaptic AMPK→p21-Activated Kinase Pathway Drives Fasting-Induced Synaptic Plasticity in AgRP Neurons
Highlights
•Fasting stimulates AMPK activity in hypothalamic AgRP neurons
•AMPK in AgRP neurons is necessary and sufficient for fasting synaptic plasticity
•AMPK phosphorylates PAK and activates PAK signaling pathway both in vitro and in vivo
•AMPK-PAK signaling in AgRP neurons is required for fasting-induced synaptic plasticity
Summary
AMP-activated protein kinase (AMPK) plays an important role in regulating food intake. The downstream AMPK substrates and neurobiological mechanisms responsible for this, however, are ill defined. Agouti-related peptide (AgRP)-expressing neurons in the arcuate nucleus regulate hunger. Their firing increases with fasting, and once engaged they cause feeding. AgRP neuron activity is regulated by state-dependent synaptic plasticity: fasting increases dendritic spines and excitatory synaptic activity; feeding does the opposite. The signaling mechanisms underlying this, however, are also unknown. Using neuron-specific approaches to measure and manipulate kinase activity specifically within AgRP neurons, we establish that fasting increases AMPK activity in AgRP neurons, that increased AMPK activity in AgRP neurons is both necessary and sufficient for fasting-induced spinogenesis and excitatory synaptic activity, and that the AMPK phosphorylation target mediating this plasticity is p21-activated kinase. This provides a signaling and neurobiological basis for both AMPK regulation of energy balance and AgRP neuron state-dependent plasticity.
“A Postsynaptic AMPK→p21-Activated Kinase Pathway Drives Fasting-Induced Synaptic Plasticity in AgRP Neurons” by Dong Kong, Yossi Dagon, John N. Campbell, Yikun Guo, Zongfang Yang, Xinchi Yi, Pratik Aryal, Kerry Wellenstein, Barbara B. Kahn, Bernardo L. Sabatini, and Bradford B. Lowell in Neuron. Published online June 16 2016 doi:10.1016/j.neuron.2016.05.025






