Princeton University researchers have found that a family of proteins with important roles in the immune system may be responsible for fine-tuning a person’s motor control as they grow, and for their gradual loss of muscle function as they age. The research potentially reveals a biological cause of weakness and instability in older people, as well as a possible future treatment that would target the proteins specifically.
The researchers reported in the journal Brain, Behavior, and Immunity that proteins in the family MHCI, or major histocompatibility complex class I, “prune” the connections, or synapses, between motor neurons and muscle fibers. Pruning is necessary during early development because at birth each muscle fiber in humans, mice and other vertebrates receives signals from dozens of neural connections. Proper motor control, however, requires that each muscle fiber receive signals from only a single motor neuron, so without the pruning carried out by MHCI proteins, fine motor control would never emerge.
But the researchers also found that MHCI levels can rise again in old age, and that the proteins may resume pruning nerve-muscle synapse, except that in a mature organism there are no extra synapses. The result is that individual muscle fibers become completely “denervated,” or detached from nervous system control. Denervated muscle fibers cannot be recruited during muscle contraction, which can leave older people weaker and more susceptible to devastating falls, making independent living difficult.
However, the Princeton researchers discovered that when MHCI levels were reduced in mice, denervation during aging was largely prevented. These findings could help scientists identify and treat the neurological causes of denervation and muscle weakness in the elderly.
Corresponding author Lisa Boulanger, an assistant professor in the Princeton Neuroscience Institute, explained that in infants, motor neurons initially make far too many connections to muscle fibers, which is part of why infants lack fine motor control. Synapse overproduction followed by pruning occurs in many different regions of the vertebrate nervous system, and the neuromuscular junction has often been used as a model for studying this process.
It is not known why more synapses are made during development than are needed. One possibility is that it allows the wiring diagram of the nervous system to be precisely tuned based on the way the circuit is used, Boulanger said. MHCI proteins help limit the final number of connections so that communication between neurons and muscles is more precise and efficient than would be possible using just a molecular code that produced a set number of connections.
“Molecules might get you to the right zip code, but pruning can make sure you arrive at the right house,” Boulanger said. “During development, it’s essential to get rid of extra synapses. But when you up-regulate MHCI when you’re older and start pruning synapses again, but you don’t have any extras to replace them.”
Boulanger worked with first author Mazell Tetruashvily, who received her doctorate in molecular biology from Princeton in 2015 and is now completing her M.D. training at University of Medicine and Dentistry of New Jersey; Marin McDonald, who received her doctorate in neuroscience from the University of California-San Diego (UCSD) in 2010, and is now a radiology resident at UCSD; and Karla Frietze, a doctoral student in Princeton’s Department of Molecular Biology. Boulanger was on the UCSD faculty before moving her lab to Princeton in 2009.
In the immune system, MHCI proteins present protein fragments, or peptides, to T cells, which are white blood cells with a central role in the body’s response to infection. This peptide presentation allows T cells to recognize and kill infected and cancerous cells, which present abnormal or foreign peptides on their MHCI proteins. It is unknown if the proteins’ ability to help recognize and destroy infected or cancerous cells is mechanistically related to the proteins’ ability to help eliminate excess synapses that the Princeton researchers discovered.
In the nervous system, MHCI proteins stop pruning synapses early in life. Why they may resume their synapse-eliminating activity later in life is unknown, Boulanger said. As immune-system proteins, MHCI levels increase with inflammation, she said. Aging is associated with chronic inflammation, which could explain the observed increase in MHCI levels and the reactivation of its former role.
The Princeton researchers found that mice bred to express less MHCI proteins had “more youthful” patterns of muscle innervation, since they were protected from denervation as they aged, Boulanger said. The mice actually lacked a protein known as beta-2 microglobulin, which forms a complex with MHCI and is necessary for MHCI expression on the surface of cells. This could be beneficial from a clinical perspective because beta-2 microglobulin is a soluble protein and can be removed from the blood, Boulanger said.
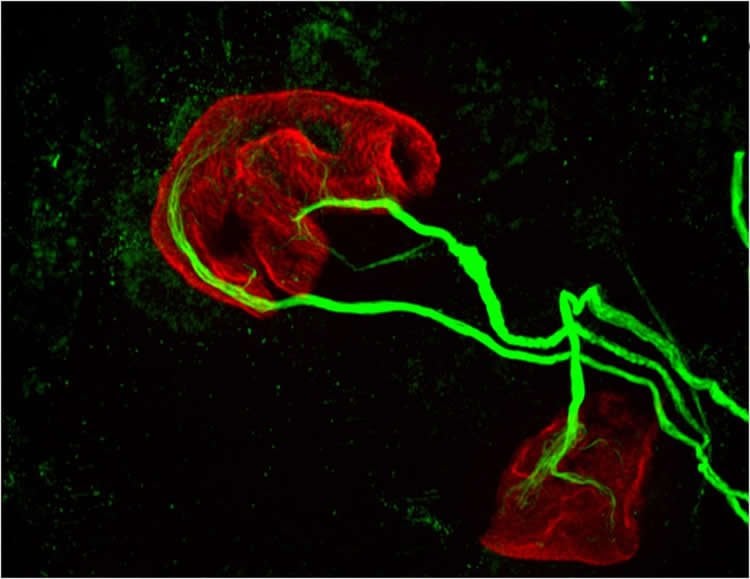
“If a rise in MHCI is the problem, having less beta-2 microglobulin might be protective,” Boulanger said. Recent results from a lab at Stanford University showed that reducing beta-2 microglobulin also helped with cognitive aging because of its effects on MHCI proteins. “Our studies raise the possibility that targeting one protein could help with both motor and cognitive aspects of aging,” Boulanger said.
Because MHCI proteins are important in the immune system, however, such an approach could result in compromised immunity, Boulanger said. The mice bred to not express beta-2 microglobulin had weakened immune systems, as a result of their lower levels of MHCI proteins. Future work will include exploring the effectiveness of other approaches to reducing the proteins’ synapse-eliminating activity in older nervous systems, ideally while leaving their immune functions intact, she said.
Funding: The research was supported by the Princeton Department of Molecular Biology and the Princeton Neuroscience Institute (grant no. 1F30AG046044-01A1), the UCSD School of Medicine, the Alfred P. Sloan Foundation, the Whitehall Foundation, and the Princeton Neuroscience Institute Innovation Fund.
Source: Morgan Kelly – Princeton University
Image Credit: Image is credited to Lisa Boulanger, Princeton Neuroscience Institute, and Mazell Tetruashvily, Department of Molecular Biology.
Original Research: Abstract for “MHCI promotes developmental synapse elimination and aging-related synapse loss at the vertebrate neuromuscular junction” by Mazell M. Tetruashvily, Marin A. McDonald, Karla K. Frietze and Lisa M. Boulanger in Brain, Behavior and Immunity. Published online Apil 8 2016 doi:10.1016/j.bbi.2016.01.008
Abstract
MHCI promotes developmental synapse elimination and aging-related synapse loss at the vertebrate neuromuscular junction
Synapse elimination at the developing neuromuscular junction (NMJ) sculpts motor circuits, and synapse loss at the aging NMJ drives motor impairments that are a major cause of loss of independence in the elderly. Here we provide evidence that at the NMJ, both developmental synapse elimination and aging-related synapse loss are promoted by specific immune proteins, members of the major histocompatibility complex class I (MHCI). MHCI is expressed at the developing NMJ, and three different methods of reducing MHCI function all disrupt synapse elimination during the second postnatal week, leaving some muscle fibers multiply-innervated, despite otherwise outwardly normal synapse formation and maturation. Conversely, overexpressing MHCI modestly accelerates developmental synapse elimination. MHCI levels at the NMJ rise with aging, and reducing MHCI levels ameliorates muscle denervation in aged mice. These findings identify an unexpected role for MHCI in the elimination of neuromuscular synapses during development, and indicate that reducing MHCI levels can preserve youthful innervation of aging muscle.
“MHCI promotes developmental synapse elimination and aging-related synapse loss at the vertebrate neuromuscular junction” by Mazell M. Tetruashvily, Marin A. McDonald, Karla K. Frietze and Lisa M. Boulanger in Brain, Behavior and Immunity. Published online Apil 8 2016 doi:10.1016/j.bbi.2016.01.008






