Summary: Researchers report altering synaptic plasticity leads to a computational switch in a hippocampal synapse which turns the presynaptic neuron turns into “detonator” mode, causing it to fire more readily.
Source: IST Austria.
Altering synaptic plasticity leads to a computational switch in a hippocampal synapse: the presynaptic neuron turns into “detonator” mode, causing its postsynaptic partner to fire more readily. This new insight into information processing in the brain by Nicholas Vyleta, previously a postdoc at IST Austria and now at Oregon Health & Science University, Carolina Borges-Merjane, postdoc at IST Austria, and Peter Jonas, Professor at IST Austria, was published in the open-access journal eLife on October 25.
Synapses form connections between neurons. Their network is intricate: a single postsynaptic neuron can be connected with thousands of presynaptic neurons. At the synapse, information is transferred from the presynaptic to the postsynaptic neuron. A key factor in information processing is the strength of the synapse. The neuromuscular junction, the connection between neuron and muscle, and the calyx of Held, a synapse in the auditory brainstem, are strong synapses. Also called full detonators, when one of these presynaptic neurons sends out a single activating signal or action potential, the postsynaptic neuron fires.
Whether such detonators also exist in the cortex of the brain has been unclear. In the present study, the authors investigate the synapse between granule cells and CA3 pyramidal cells in the hippocampus, using a recently developed method to simultaneously stimulate the individual presynaptic terminal and record from the connected postsynaptic CA3 neuron. Under normal conditions, a single action potential from the granule cell does not induce firing in the CA3 neuron. Instead, the granule cells are ‘conditional detonators’: burst firing of action potentials from a granule cell is required to get the CA3 neuron to fire as well.
But when the researchers altered the synaptic plasticity of the granule cell by stimulating a single presynaptic terminal to fire at high frequency for only one second, causing post-tetanic potentiation (PTP), the picture changes. The granule cell turns into a full detonator: a single action potential causes the CA3 neuron to fire. Peter Jonas explains the significance of their study: “It is generally thought that individual synapses in the brain are weak, and that tens or hundreds of inputs have to be integrated to activate a neuron. The present paper challenges this view, showing that full detonator synapses exist in the brain. This has important implications for higher order computations in the circuit.”
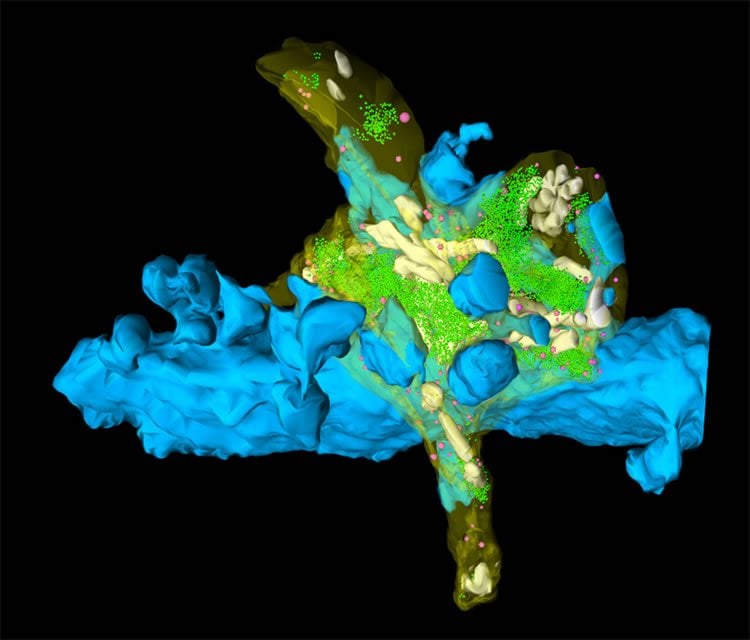
Short-term synaptic plasticity in the form of PTP produces a computational switch at the studied synapse. This change is prolonged, lasting for tens of seconds. This could be critical for information coding, storage and recall in the network formed by granule cells and CA3 neurons, as Nicholas Vyleta points out: “A single hippocampal mossy fiber synapse can produce an action potential in a postsynaptic pyramidal neuron after activity-dependent enhancement of transmitter release. This computational switch may allow a single piece of highly specific information from the dentate gyrus to be transmitted through the hippocampal formation, and may form the basis of the processing of information in this circuit by pattern separation.”
The study was published in eLife, an open access journal for the life sciences and biomedicine. The journal is supported by the Howard Hughes Medical Institute, the Max Planck Society and the Wellcome Trust. For Carolina Borges-Merjane and Nicholas Vyleta, publishing their new findings in the open access journal provides several advantages: “Publishing our work in eLife maximizes the impact of our research and availability to other scientists. eLife has already been well recognized as a reliable journal, with efficient peer-review by scientists from many fields. Our experiences with reviewers and the editor were fair, efficient and their comments were very constructive. Plus the paper is available freely not just to other scientists, but also the general public, including our families. “
Source: IST Austria
Image Source: NeuroscienceNews.com image is credited to the researchers/IST Austria.
Original Research: Full open access research for “Plasticity-dependent, full detonation at hippocampal mossy fiber–CA3 pyramidal neuron synapses” by Nicholas P Vyleta, Carolina Borges-Merjane, and Peter Jonas in eLife. Published online October 25 2016 doi:10.7554/eLife.17977
[cbtabs][cbtab title=”MLA”]IST Austria “Full Detonation in the Hippocampus.” NeuroscienceNews. NeuroscienceNews, 12 November 2016.
<https://neurosciencenews.com/synaptic-plasticity-hippocampus-5497/>.[/cbtab][cbtab title=”APA”]IST Austria (2016, November 12). Full Detonation in the Hippocampus. NeuroscienceNew. Retrieved November 12, 2016 from https://neurosciencenews.com/synaptic-plasticity-hippocampus-5497/[/cbtab][cbtab title=”Chicago”]IST Austria “Full Detonation in the Hippocampus.” https://neurosciencenews.com/synaptic-plasticity-hippocampus-5497/ (accessed November 12, 2016).[/cbtab][/cbtabs]
Abstract
Plasticity-dependent, full detonation at hippocampal mossy fiber–CA3 pyramidal neuron synapses
Mossy fiber synapses on CA3 pyramidal cells are ‘conditional detonators’ that reliably discharge postsynaptic targets. The ‘conditional’ nature implies that burst activity in dentate gyrus granule cells is required for detonation. Whether single unitary excitatory postsynaptic potentials (EPSPs) trigger spikes in CA3 neurons remains unknown. Mossy fiber synapses exhibit both pronounced short-term facilitation and uniquely large post-tetanic potentiation (PTP). We tested whether PTP could convert mossy fiber synapses from subdetonator into detonator mode, using a recently developed method to selectively and noninvasively stimulate individual presynaptic terminals in rat brain slices. Unitary EPSPs failed to initiate a spike in CA3 neurons under control conditions, but reliably discharged them after induction of presynaptic short-term plasticity. Remarkably, PTP switched mossy fiber synapses into full detonators for tens of seconds. Plasticity-dependent detonation may be critical for efficient coding, storage, and recall of information in the granule cell–CA3 cell network.
“Plasticity-dependent, full detonation at hippocampal mossy fiber–CA3 pyramidal neuron synapses” by Nicholas P Vyleta, Carolina Borges-Merjane, and Peter Jonas in eLife. Published online October 25 2016 doi:10.7554/eLife.17977