Summary: Mouse study reveals pain-sensing neurons also help fight skin infections and help prevent its spread. The findings suggest a new type of immunity.
Source: University of Pittsburgh
Pinch yourself. If you feel pain, it’s thanks to specialized nerve endings in the skin. And, in a surprising discovery, researchers at the University of Pittsburgh School of Medicine demonstrated that pain-sensing nerves also help fight skin infections and prevent its spread, suggesting a new type of immunity. The findings, based on studies in mice, were published today in the journal Cell.
“These pain-sensing nerves can detect pathogens, and for the first time, we’ve shown that they activate an immune response and also signal protective immunity in sites adjacent to the infection,” said Daniel Kaplan, M.D., Ph.D., professor of dermatology and immunology at Pitt’s School of Medicine and the senior author of the study. “This demonstrates that the immune and nervous systems work synergistically for host defense. These findings also could have important implications for developing more specific therapies for autoimmune skin diseases like psoriasis.”
Until about a decade ago, pain was thought to have evolved as a way for your body to tell you to stay away from a particular stimulus or to signal a problem with its function, like an injury. More recently, however, researchers have shown that it may play an important role in immunity against some pathogens.
In the study, Kaplan and first author Jonathan Cohen, an M.D./Ph.D. student in Kaplan’s lab, collaborated with Pitt neurobiology professors and pain experts Kathy Albers, Ph.D., and Brian Davis, Ph.D., to develop an optogenetic mouse model where pain-sensing neurons in the skin could be activated by shining blue light.
They first showed that just activating these neurons released a small protein called CGRP, which recruited different types of immune cells to the site. This suggested that neurons detecting skin pathogens on their own kickstart an immune response even before sentry immune cells could.
Then in the same mouse model, they infected the animals with either Candida albicans, a fungus that causes candidiasis, commonly known as thrush, or Staphylococcs aureus, a common bacterium that can turn deadly under certain conditions.
Using optogenetics and chemical nerve blockers, the researchers showed through a series of elegant experiments that when the fungus infected the skin at one location, the nerves not only detected and initiated an immune response to fight the infection, but also sent a signal toward the spinal cord. Those signals then boomeranged back to skin at areas around the infection to activate immune defenses in anticipation, thereby preventing the infection from spreading.
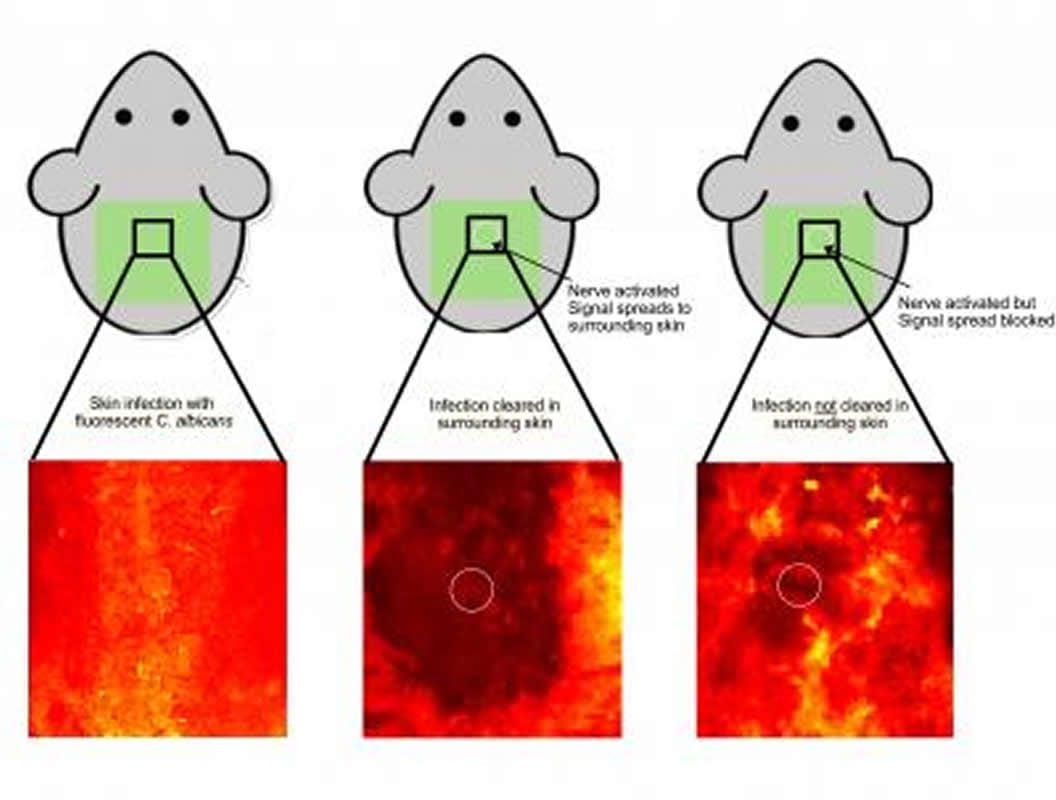
The researchers called this new nerve-driven protective mechanism “anticipatory immunity.”
“The advantage of involving the nervous system is that it can communicate information across a space in a span of milliseconds, compared to hours or days for the immune cells to do the same function,” said Jonathan Cohen, an M.D./Ph.D. student in Kaplan’s lab and the first author of the study.
“It’s the difference between sending Paul Revere to warn of the British advance and sending a telegram to do the same.”
Kaplan says that while it remains to be seen how the findings translate to humans, they have interesting implications for autoimmune diseases of barrier tissues like the skin or gut.
“Understanding this really new type of immunity raises the intriguing question of whether we could develop a drug to selectively suppress excessive autoimmune inflammation in specific tissues, avoiding the negative side effects that come with using a broad immunosuppressant that affects the entire body,” he says.
Additional authors on the study included Tara N. Edwards, Ph.D., Andrew W. Liu, Toshiro Hirai, Ph.D., Marsha Ritter Jones, M.D., Ph.D., Yao Li, Ph.D., Shiqun Zhang, Ph.D., Jonhan Ho, M.D., Brian M. Davis, Ph.D., and Kathryn M. Albers, Ph.D., all of Pitt; and Jianing Wu of Tsinghua University in China.
Funding: The study was supported by National Institutes of Health grants 1S10OD011925-01, T32AI060525, R01AR071720 and a grant from Pitt’s Department of Anesthesiology and Perioperative Medicine.
Source:
University of Pittsburgh
Media Contacts:
Arvind Suresh – University of Pittsburgh
Image Source:
The image is credited to Jonathan Cohen/Adapted from Cohen et. Al., Cell 2019.
Original Research: Closed access
“Cutaneous TRPV1+ Neurons Trigger Protective Innate Type 17 Anticipatory Immunity”. Jonathan A. Cohen, Tara N. Edwards, Andrew W. Liu, Brian M. Davis, Kathryn M. Albers, Daniel H. Kaplan.
Cell. doi:10.1016/j.cell.2019.06.022
Abstract
Cutaneous TRPV1+ Neurons Trigger Protective Innate Type 17 Anticipatory Immunity
Highlights
• Cutaneous TRPV1 + neuron activation is sufficient to initiate type 17 inflammation
• Cutaneous TRPV1 + neuron activation augments local host defense
• Type 17 innate immunity via nerve reflex provides regional anticipatory immunity
Summary
Cutaneous TRPV1 + neurons directly sense noxious stimuli, inflammatory cytokines, and pathogen-associated molecules and are required for innate immunity against some skin pathogens. Important unanswered questions are whether TRPV1 + neuron activation in isolation is sufficient to initiate innate immune responses and what is the biological function for TRPV1 + neuron-initiated immune responses. We used TRPV1-Ai32 optogenetic mice and cutaneous light stimulation to activate cutaneous neurons in the absence of tissue damage or pathogen-associated products. We found that TRPV1 + neuron activation was sufficient to elicit a local type 17 immune response that augmented host defense to C. albicans and S. aureus. Moreover, local neuron activation elicited type 17 responses and augmented host defense at adjacent, unstimulated skin through a nerve reflex arc. These data show the sufficiency of TRPV1 + neuron activation for host defense and demonstrate the existence of functional anticipatory innate immunity at sites adjacent to infection that depends on antidromic neuron activation.







