Virginia Bioinformatics Institute researchers find possible cancer markers in neural crest.
Researchers at the Virginia Bioinformatics Institute have uncovered a link between the genomes of cells originating in the neural crest and development of tumors — a discovery that could lead to new ways to diagnose and treat cancer.
The new finding, recently published in Oncotarget, resolves why some cancer types share genomic and clinical features.
The discovery may also lead to new ways to diagnose and treat brain cancer, such as gliomas, medulloblastomas, and neuroblastomas; and skin cancer, known as melanoma.
More than 22,000 new cases of brain cancer and more than 73,000 new cases of skin cancer and were expected to arise in Americans in 2015, according to the National Cancer Institute.
To reveal when cancer-causing genomic changes occur , a research group led by Harold “Skip” Garner, a professor in the departments of biological science, computer science, and basic science at Virginia Tech Carilion Medical School, analyzed an often ignored part of the human genome — repetitive DNA sequences referred to as microsatellites.
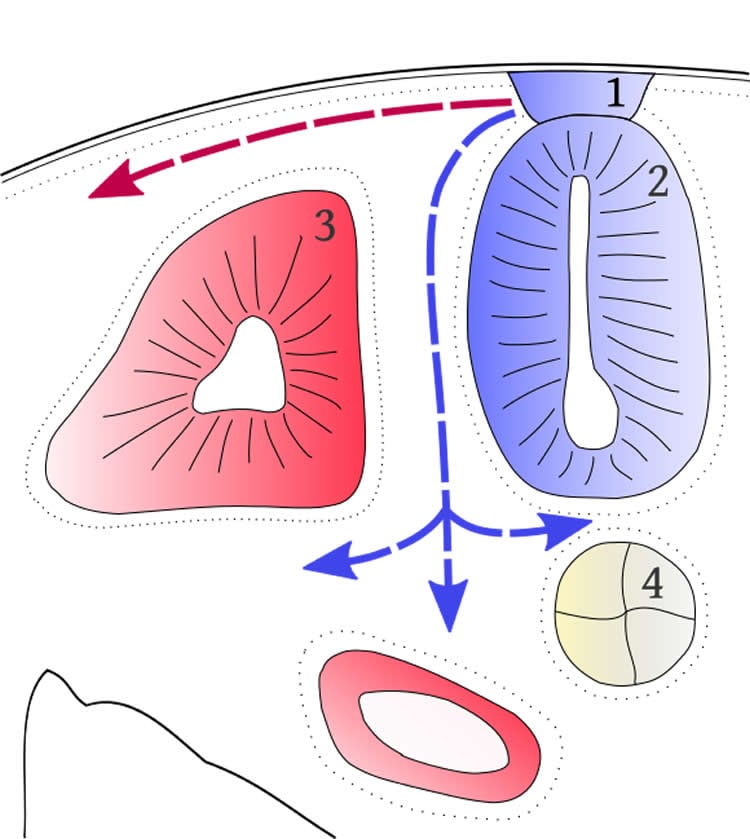
Over 1 million microsatellites exist in the human genome, including in neural crest tissues, a thin layer of cells within an embryo that contains genetic instructions to build hundreds of cell types, from neurons to adrenal cells.
When cells migrate from the neural crest, researchers say the instructions may become garbled, causing cancer cells to emerge. Neurological tumors, for example, may arise from glial cells that develop from the crest.
Researchers with the institute’s Medical Informatics Systems Division say cancer types can be found or predicted from specific markers within these repetitive sequences, known as cancer-associated microsatellite loci, or CAML.
Long considered “junk DNA” or “dark matter” within the genome because their function was unclear, microsatellites are known for their role in certain diseases such as Fragile X and Huntington’s disease.
Garner’s group has shown that these regions can be informative about diseases ranging from cancer to autism spectrum disorder.
With more study, researchers believe interrelated hereditary and genetic traits of certain cancers can be traced to their common origin at the neural crest, leading to potentially better therapies and easier tumor identification.
The findings have been licensed to Genomeon, a company co-founded by Garner to develop new ways to assess cancer risk, create diagnostics, and explore potential drug targets to help cancer patients.
Source: John Pastor – Virginia Tech
Image Credit: The image is in the public domain
Original Research: Abstract for “‘Cut from the same cloth’: Shared microsatellite variants among cancers link to ectodermal tissues-neural tube and crest cells” by Enusha Karunasena, Lauren J. Mciver, Jasmin H. Bavarva, Xiaowei Wu, Hongxiao Zhu, and Harold R. Garner in Oncotarget. Published online June 17 2015 doi:not available
Abstract
‘Cut from the same cloth’: Shared microsatellite variants among cancers link to ectodermal tissues-neural tube and crest cells
The pluripotent cells of the embryonic ectodermal tissues are known to be a precursor for multiple tumor types. The adaptability of these cells is a trait exploited by cancer. We previously described cancer-associated microsatellite loci (CAML) shared between glioblastoma (GBM) and lower-grade gliomas. Therefore, we hypothesized that these variants, identified from germline DNA, are shared by cancers from tissues originating from ectodermal tissues: neural tube cells (NTC) and crest cells (NCC). Using exome sequencing data from four cancers with origins to NTC and NCC, a ‘signature’ of loci significant to each cancer (p-value ≤ 0.01) was created and compared with previously identified CAML from breast cancer. The results of this analysis show that variant loci among the cancers with tissue origins from NTC/NCC were closely linked. Signaling pathways linked to genes with non-coding CAML genotypes revealed enriched connections to hereditary, neurological, and developmental disease or disorders. Thus, variants in genes from tissues initiating from NTC/NCC, if recurrently detected, may indicate a common etiology. Additionally, CAML genotypes from non-tumor DNA may predict cancer phenotypes and are common to shared embryonic tissues of origin.
“‘Cut from the same cloth’: Shared microsatellite variants among cancers link to ectodermal tissues-neural tube and crest cells” by Enusha Karunasena, Lauren J. Mciver, Jasmin H. Bavarva, Xiaowei Wu, Hongxiao Zhu, and Harold R. Garner in Oncotarget. Published online June 17 2015 doi:not available






