Summary: Researchers have identified a population of immune cells that appear to be associated with neurons which play a role in fat storage and obesity.
Source: IGC Portugal.
The biological causes underlying obesity have been under intense scrutiny with studies suggesting a link between the nervous and the immune systems. Now, in a breakthrough study to be published in Nature Medicine on 9 October, a research team led by Ana Domingos, from Instituto Gulbenkian de Ciência (IGC; Portugal), discovered an unforeseen population of immune cells associated with neurons that play a direct role in obesity.
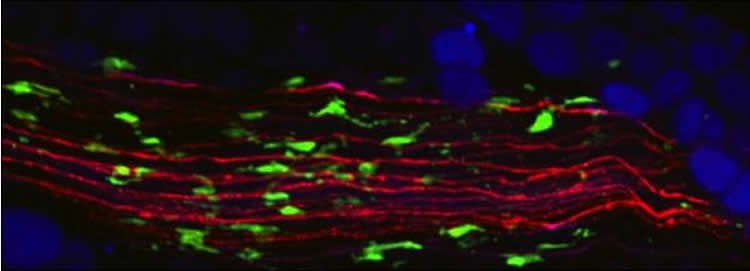
These immune cells are macrophages, a type of white blood cells responsible for inflammatory responses in the body. Previous studies had alluded to a role for macrophages in the adipose tissue inflammation that occurs in obesity, but the mechanism of action linking these cells to neurons and fat breakdown was unclear. Domingos’ team showed that specialized macrophages are in direct contact with neurons and affect neuronal activation that is critical for fat mass reduction. The team had previously discovered that adipose tissue is innervated by a set of sympathetic neurons that release norepinephrine, a neurotransmitter that induces fat breakdown. The new study shows that these sympathetic neurons are in intimate contact with a particular type of macrophage that they coined SAMs (sympathetic neuron-associated macrophages).
The researchers found that SAMs clear out norepinephrine and that obese mice had many more of these cells attached to neurons than lean mice. This means that SAMs contribute to obesity by decreasing norepinephrine content in fat, thus preventing subsequent fat reduction.
By conducting genetic studies in mice, the research team was able to pinpoint the molecular mechanism underlying SAM-mediated destruction of norepinephrine. The import mechanism of this neurotransmitter involves the transporter for norepinephrine (the protein Slc6a2) that is present in SAMs but not in other immune cells.
Furthermore, they showed that blocking the import mechanism of norepinephrine by SAMs boosts fat breakdown, energy dissipation, and weight loss. They further confirmed that SAMs and the associated molecular machinery for norepinephrine clearing also exist in humans, through analysis of human nervous system samples.
“The role of norepinephrine transporter in SAMs offers a targeted approach that may overcome the noxious off-target effects of several known drugs that block this molecular target”, says Ana Domingos.
These results set the stage for the development of new anti-obesity therapies.
Obesity is a serious health condition that affects about 13% of the world’s adult population, according to an estimate from 2014 of the World Health Organization.
This study was conducted at IGC in collaboration with the University of California (USA), Hospital de Santa Maria (Portugal), Ludwig-Maximilians Universität München (Germany), Vanderbilt University School of Medicine (USA), IRCCS San Raffaele Scientific Institute (Italy), and Curry Cabral Hospital (Portugal).
Funding: This study was funded by Fundação para a Ciência e a Tecnologia (FCT; Portugal), the European Molecular Biology Organization (EMBO), the Human Frontier Science Program (HFSP), Maratona da Saúde (Portugal), and the National Institutes of Health (NIH).
Source: Kate McAllister – IGC Portugal
Image Source: NeuroscienceNews.com image is credited to Roksana Pirzgalska, IGC.
Original Research: Abstract for “Sympathetic neuron–associated macrophages contribute to obesity by importing and metabolizing norepinephrine” by Pirzgalska, R. M., Seixas, E., Seidman, J. S., Link, V. M., Sanchez, N. M., Mahu, I., Mendes, R., Gres, V., Kubasova, N., Morris, I., Arús, B.A., Larabee, C. M., Vasques, M., Tortosa, F., Sousa, A.L., Anandan, S., Tranfield, E., Hahn, M.K., Iannacone, M., Spann, N.J., Glass, C.K., and Domingos, A.I. in Nature Medicine. Published online October 9 2019 doi:10.1038/nm.4422
[cbtabs][cbtab title=”MLA”]IGC Portugal “Making Fat Mice Lean: Specialized Macrophages Responsible For Fat Breakdown.” NeuroscienceNews. NeuroscienceNews, 9 October 2019.
<https://neurosciencenews.com/macrophages-fat-breakdown-7693/>.[/cbtab][cbtab title=”APA”]IGC Portugal (2019, October 9). Making Fat Mice Lean: Specialized Macrophages Responsible For Fat Breakdown. NeuroscienceNews. Retrieved October 9, 2019 from https://neurosciencenews.com/macrophages-fat-breakdown-7693/[/cbtab][cbtab title=”Chicago”]IGC Portugal “Making Fat Mice Lean: Specialized Macrophages Responsible For Fat Breakdown.” https://neurosciencenews.com/macrophages-fat-breakdown-7693/ (accessed October 9, 2019).[/cbtab][/cbtabs]
Abstract
Sympathetic neuron–associated macrophages contribute to obesity by importing and metabolizing norepinephrine
The cellular mechanism(s) linking macrophages to norepinephrine (NE)-mediated regulation of thermogenesis have been a topic of debate. Here we identify sympathetic neuron–associated macrophages (SAMs) as a population of cells that mediate clearance of NE via expression of solute carrier family 6 member 2 (SLC6A2), an NE transporter, and monoamine oxidase A (MAOA), a degradation enzyme. Optogenetic activation of the sympathetic nervous system (SNS) upregulates NE uptake by SAMs and shifts the SAM profile to a more proinflammatory state. NE uptake by SAMs is prevented by genetic deletion of Slc6a2 or inhibition of the encoded transporter. We also observed an increased proportion of SAMs in the SNS of two mouse models of obesity. Genetic ablation of Slc6a2 in SAMs increases brown adipose tissue (BAT) content, causes browning of white fat, increases thermogenesis, and leads to substantial and sustained weight loss in obese mice. We further show that this pathway is conserved, as human sympathetic ganglia also contain SAMs expressing the analogous molecular machinery for NE clearance, which thus constitutes a potential target for obesity treatment.
“Sympathetic neuron–associated macrophages contribute to obesity by importing and metabolizing norepinephrine” by Pirzgalska, R. M., Seixas, E., Seidman, J. S., Link, V. M., Sanchez, N. M., Mahu, I., Mendes, R., Gres, V., Kubasova, N., Morris, I., Arús, B.A., Larabee, C. M., Vasques, M., Tortosa, F., Sousa, A.L., Anandan, S., Tranfield, E., Hahn, M.K., Iannacone, M., Spann, N.J., Glass, C.K., and Domingos, A.I. in Nature Medicine. Published online October 9 2019 doi:10.1038/nm.4422






