Scientists at the UNC Eshelman School of Pharmacy are creating white blood cells that teach brain cells to heal the damage caused by degenerative neurological disorders like Parkinson’s disease.
As a potential treatment for Parkinson’s disease, scientists at the University of North Carolina at Chapel Hill have created smarter immune cells that produce and deliver a healing protein to the brain while also teaching neurons to begin making the protein for themselves.
The researchers, led by Elena Batrakova, an associate professor at the UNC Eshelman School of Pharmacy’s Center for Nanotechnology in Drug Delivery, genetically modified white blood cells called macrophages to produce glial cell-derived neurotrophic factor, or GDNF, and deliver it to the brain. Glial cells provide support and protection for nerve cells throughout the brain and body, and GDNF can heal and stimulate the growth of damaged neurons.
“Currently, there are no treatments that can halt or reverse the course of Parkinson’s disease. There are only therapies to address quality of life, such as dopamine replacement,” Batrakova said. “However, studies have shown that delivering neurotrophic factor to the brain not only promotes the survival of neurons but also reverses the progression of Parkinson’s disease.”
In addition to delivering GDNF, the engineered macrophages can “teach” neurons to make the protein for themselves by delivering both the tools and the instructions needed: DNA, messenger RNA and transcription factor.
Successfully delivering the treatment to the brain is the key to the success of GDNF therapy, said Batrakova. Using immune cells avoids the body’s natural defenses. The repurposed macrophages are also able to penetrate the blood-brain barrier, something most medicines cannot do. The reprogrammed cells travel to the brain and produce tiny bubbles called exosomes that contain GDNF. The cells release the exosomes, which then are able to deliver the proteins to neurons in the brain. The work is described in an article published online by PLOS ONE.
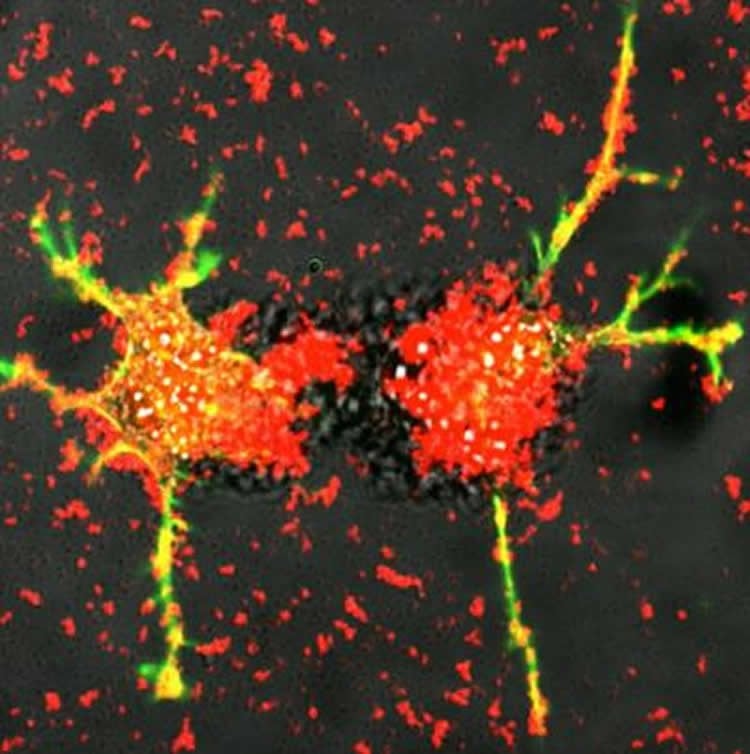
“By teaching immune system cells to make this protective protein, we harness the natural systems of the body to combat degenerative conditions like Parkinson’s disease,” Batrakova said.
The North Carolina Biotechnology Center awarded a $50,000 Technology Enhancement Grant to the School to help develop the technology into a viable treatment that can be licensed and commercialized.
“This award is an enormously important step towards further successful commercialization of our very exciting cell technologies,” said Alexander Kabanov, director of the nanotechnology center. “We will continue our translational efforts at CNDD, and very soon I believe we will see these discoveries on the frontiers of scientific moving into clinical practice.”
Funding: The research was supported by the National Institutes of Health, US Department of Defense, and Russian Ministry of Science and Education.
Source: University of North Carolina at Chapel Hill
Image Source: The image is credited to Elena Batrakova/UNC Eshelman School Of Pharmacy
Original Research: Full open access research for “GDNF-Transfected Macrophages Produce Potent Neuroprotective Effects in Parkinson’s Disease Mouse Model” by Yuling Zhao, Matthew J. Haney, Richa Gupta, John P. Bohnsack, Zhijian He, Alexander V. Kabanov, and Elena V. Batrakova in PLOS ONE. Published online September 2015 doi:10.1371/journal.pone.0106867
Abstract
GDNF-Transfected Macrophages Produce Potent Neuroprotective Effects in Parkinson’s Disease Mouse Model
The pathobiology of Parkinson’s disease (PD) is associated with the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) projecting to the striatum. Currently, there are no treatments that can halt or reverse the course of PD; only palliative therapies, such as replacement strategies for missing neurotransmitters, exist. Thus, the successful brain delivery of neurotrophic factors that promote neuronal survival and reverse the disease progression is crucial. We demonstrated earlier systemically administered autologous macrophages can deliver nanoformulated antioxidant, catalase, to the SNpc providing potent anti-inflammatory effects in PD mouse models. Here we evaluated genetically-modified macrophages for active targeted brain delivery of glial cell-line derived neurotropic factor (GDNF). To capitalize on the beneficial properties afforded by alternatively activated macrophages, transfected with GDNF-encoded pDNA cells were further differentiated toward regenerative M2 phenotype. A systemic administration of GDNF-expressing macrophages significantly ameliorated neurodegeneration and neuroinflammation in PD mice. Behavioral studies confirmed neuroprotective effects of the macrophage-based drug delivery system. One of the suggested mechanisms of therapeutic effects is the release of exosomes containing the expressed neurotropic factor followed by the efficient GDNF transfer to target neurons. Such formulations can serve as a new technology based on cell-mediated active delivery of therapeutic proteins that attenuate and reverse progression of PD, and ultimately provide hope for those patients who are already significantly disabled by the disease.
“GDNF-Transfected Macrophages Produce Potent Neuroprotective Effects in Parkinson’s Disease Mouse Model” by Yuling Zhao, Matthew J. Haney, Richa Gupta, John P. Bohnsack, Zhijian He, Alexander V. Kabanov, and Elena V. Batrakova in PLOS ONE. Published online September 2015 doi:10.1371/journal.pone.0106867