Summary: Researchers report a protein normally associated with prion diseases may help to maintain myelin.
Source: WUSTL.
Protein helps maintain nerve function over lifespan.
Scientists have clarified details in understanding the beneficial function of a type of protein normally associated with prion diseases of the brain, such as bovine spongiform encephalopathy (commonly known as mad cow disease) and its human counterpart, variant Creutzfeldt-Jakob disease.
Studying mice and zebrafish, researchers from Washington University School of Medicine in St. Louis and the University of Zurich have shown that the proteins — when properly folded — play a vital role in nerve cell function by maintaining the insulation around axons, the nervous system’s electrical “wiring.”
The study appears August 8 in the journal Nature.
Improperly formed prion proteins that cause disease are infectious because they hijack their neighbors, resulting in misfolded proteins and setting off a domino effect that spreads through the brain destroying tissue. Although the role of prion proteins in these fatal brain diseases is well-known, scientists have long puzzled over the normal function of the protein, called PrPC.
“Previous studies have suggested a role for prion proteins in maintaining neurons, but until now, no one knew how the properly folded versions of the proteins function,” said co-author Kelly R. Monk, PhD, an associate professor of developmental biology at Washington University. “It’s surprising to see that the protein has a role in maintaining the structure of nerve cells, considering that a misfolded version of PrPC is known to cause fatal brain diseases.”
Past work by the researchers at the University of Zurich demonstrated that mice lacking PrPC had disruptions in the insulation surrounding axons, but the reasons for the disruptions were unclear. The new study demonstrates that PrPC binds to Schwann cells, which are cells that provide support for the brain’s neurons. Schwann cells produce the nerve-insulating protein called myelin and then wrap this insulation around the long, thin axons. Properly insulated axons enable the rapid propagation of nerve signals. Specifically, PrPC binds to a docking site on Schwann cells called Gpr126.
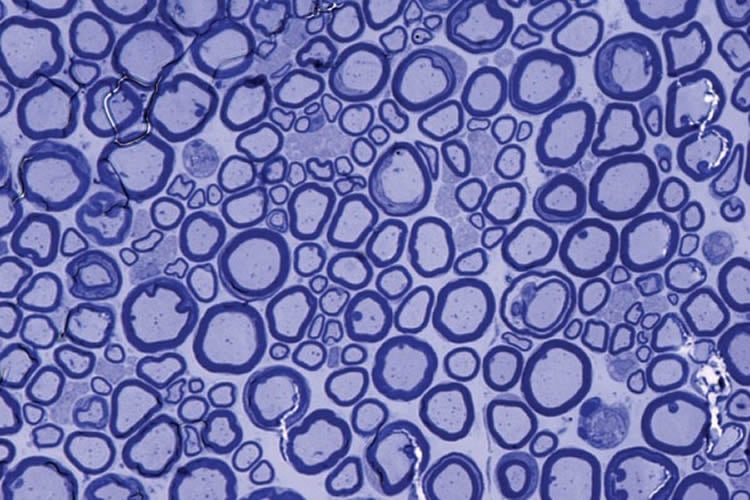
In past work, Monk and her Washington University colleagues demonstrated that the docking site on cells played an important role in nerve formation during embryonic development in zebrafish and in mice. But the new study identifies roles for both Gpr126 and PrPC in maintaining the integrity of neurons through adulthood.
When either of these components is missing, Monk said mice experience a gradual loss of interactions between Schwann cells and axons, with a resulting loss of of myelin. Without this important insulation, walking progressively becomes more difficult for mice, and they eventually reach a state of paralysis.
“We have identified a definitive function for the normal prion protein and clarified how it works on a molecular level,” said senior author Adriano Aguzzi, MD, PhD, of the University of Zurich. “Our study answers a question that has been intensely researched since the prion gene’s discovery in 1985.”
The researchers said the findings may have implications for understanding and eventually treating nerve disorders that result from the loss of the insulating myelin sheaths, such as Charcot-Marie-Tooth disease and other devastating peripheral neuropathies.
Funding: This work was supported by the European Research Council; a European Union Framework 7 Grant (NEURINOX); the Swiss National Foundation; the Clinical Research Priority Programs “Small RNAs” and “Human Hemato-Lymphatic Diseases;” SystemsX.ch; the Novartis Research Foundation; the Swiss National Science Foundation; the Synapsis Foundation; and the National Institutes of Health (NIH), grant numbers F32 NS087786 and NS079445.
Source: Diane Duke Williams – WUSTL
Image Source: This NeuroscienceNews.com image is credited to Amit Mogha.
Original Research: Abstract for “The prion protein is an agonistic ligand of the G protein-coupled receptor Adgrg6” by Alexander Küffer, Asvin K. K. Lakkaraju, Amit Mogha, Sarah C. Petersen, Kristina Airich, Cédric Doucerain, Rajlakshmi Marpakwar, Pamela Bakirci, Assunta Senatore, Arnaud Monnard, Carmen Schiavi, Mario Nuvolone, Bianka Grosshans, Simone Hornemann, Frederic Bassilana, Kelly R. Monk and Adriano Aguzzi in Nature. Published online August 8 2016 doi:10.1038/nature19312
[cbtabs][cbtab title=”MLA”]WUSTL. “Beneficial Role Clarified for Brain Protein Associated with Mad Cow Disease.” NeuroscienceNews. NeuroscienceNews, 8 August 2016.
<https://neurosciencenews.com/cjd-prions-function-neurology-4796/>.[/cbtab][cbtab title=”APA”]WUSTL. (2016, August 8). Beneficial Role Clarified for Brain Protein Associated with Mad Cow Disease. NeuroscienceNew. Retrieved August 8, 2016 from https://neurosciencenews.com/cjd-prions-function-neurology-4796/[/cbtab][cbtab title=”Chicago”]WUSTL. “Beneficial Role Clarified for Brain Protein Associated with Mad Cow Disease.” https://neurosciencenews.com/cjd-prions-function-neurology-4796/ (accessed August 8, 2016).[/cbtab][/cbtabs]
Abstract
The prion protein is an agonistic ligand of the G protein-coupled receptor Adgrg6
Ablation of the cellular prion protein PrPC leads to a chronic demyelinating polyneuropathy affecting Schwann cells. Neuron-restricted expression of PrPC prevents the disease1, suggesting that PrPC acts in trans through an unidentified Schwann cell receptor. Here we show that the cAMP concentration in sciatic nerves from PrPC-deficient mice is reduced, suggesting that PrPC acts via a G protein-coupled receptor (GPCR). The amino-terminal flexible tail (residues 23–120) of PrPC triggered a concentration-dependent increase in cAMP in primary Schwann cells, in the Schwann cell line SW10, and in HEK293T cells overexpressing the GPCR Adgrg6 (also known as Gpr126). By contrast, naive HEK293T cells and HEK293T cells expressing several other GPCRs did not react to the flexible tail, and ablation of Gpr126 from SW10 cells abolished the flexible tail-induced cAMP response. The flexible tail contains a polycationic cluster (KKRPKPG) similar to the GPRGKPG motif of the Gpr126 agonist type-IV collagen2. A KKRPKPG-containing PrPC-derived peptide (FT23–50) sufficed to induce a Gpr126-dependent cAMP response in cells and mice, and improved myelination in hypomorphic gpr126 mutant zebrafish (Danio rerio). Substitution of the cationic residues with alanines abolished the biological activity of both FT23–50 and the equivalent type-IV collagen peptide. We conclude that PrPC promotes myelin homeostasis through flexible tail-mediated Gpr126 agonism. As well as clarifying the physiological role of PrPC, these observations are relevant to the pathogenesis of demyelinating polyneuropathies—common debilitating diseases for which there are limited therapeutic options.
“The prion protein is an agonistic ligand of the G protein-coupled receptor Adgrg6” by Alexander Küffer, Asvin K. K. Lakkaraju, Amit Mogha, Sarah C. Petersen, Kristina Airich, Cédric Doucerain, Rajlakshmi Marpakwar, Pamela Bakirci, Assunta Senatore, Arnaud Monnard, Carmen Schiavi, Mario Nuvolone, Bianka Grosshans, Simone Hornemann, Frederic Bassilana, Kelly R. Monk and Adriano Aguzzi in Nature. Published online August 8 2016 doi:10.1038/nature19312