Summary: Researchers have identified the structure of the human type 2 cannabinoid receptor. The findings, researchers say, can help in the development of new treatments for inflammatory and neurodegenerative diseases.
Source: Moscow Institute of Physics and Technology.
A Chinese research team joined forces with Russian and U.S. biologists to obtain the crystal structure of the human type 2 cannabinoid receptor. Their findings will help develop drugs against inflammatory, neurodegenerative, and other diseases. The authors of the paper in Cell compare the newly discovered structure to that of the type 1 cannabinoid receptor, deeming the two receptors to be the “yin and yang” of the human endocannabinoid system.
Drugs rely on knowing receptor structure
The two cannabinoid receptors, CB1 and CB2, belong to the so-called endocannabinoid system. This refers to a signaling system in the human body that regulates biological processes such as metabolism, pain sensation, neuronal activity, immune function, and so on. It has been shown that the cannabinoid receptors can be targeted to alleviate certain pathological conditions, including chronic pain.
While the CB1 receptors are mostly found in the nervous system and are responsible for psychoactive effects, the CB2 receptors are predominantly present in the immune system. Studies indicate that CB2 is a promising target for immunotherapy, as well as treating inflammatory and neuropathic pain, and neurodegenerative diseases. It has also been shown that molecules blocking CB2 can reduce tumor growth.
To effectively treat pathological conditions, drugs need to specifically target CB1 or CB2. However, the two receptors are very much alike. The amino acid sequences that encode them are 44 percent identical. So developing a selective medicine requires knowing the structure of both targets in great detail. Unlike CB1, the structure of CB2 has remained unknown up until now.
X-raying CB2 crystal reveals its structure
To identify the shape of an individual molecule, researchers make a crystal from many such molecules. When arranged in this highly ordered way, the molecules can be exposed to X-rays, revealing their structure.
The team made a crystal from CB2 receptors bound to molecules blocking this receptor, which are potential drug candidates. That way, the X-ray analysis allowed the team to see both the structure of CB2 and how it connects to the blocking molecule, or antagonist.
However, receptors are unstable proteins by nature. To study them, they need to be modified using gene engineering. This involves introducing mutations that make the protein stable without changing its structure or function.
CompoMug is a software suite that predicts mutations potentially useful for stabilizing receptor molecules. The mutations then need to be tested experimentally. This software was developed by two researchers from the Moscow Institute of Physics and Technology and the University of Southern California: visiting MIPT professor Vsevolod Katritch and Petr Popov from the Laboratory of Structural Biology of G Protein-Coupled Receptors.
In the study, the CB2 receptor had five mutations prompted by CompoMug.
Receptor structure discovery enables smarter drugs
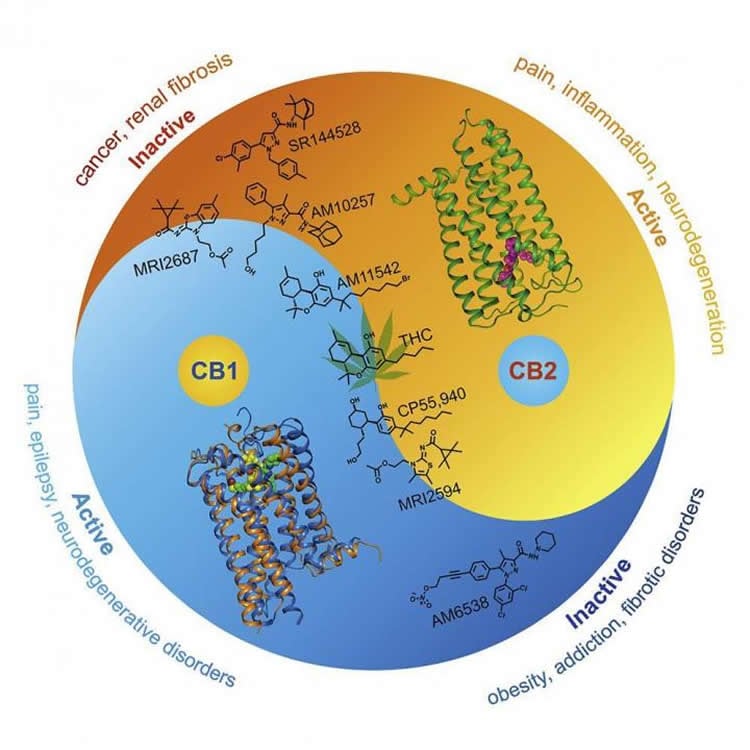
The researchers contrasted the structure of CB2 with that of CB1. They concluded that substances activating one of the receptors can actually weaken or inhibit the other, and vice versa. This opens up a possibility not just for drugs that target exclusively one receptor, but those that affect both receptors, but in different ways. Figure 2 shows the therapeutic implications.
“Every G protein-coupled receptor structure that is discovered has prospects for rational design of more efficient drugs,” study co-author Petr Popov explains. “Now that the structures of both cannabinoid receptors are known, we can design selective compounds targeting only one of the receptors, as well as agents with a desired polypharmacological profile targeting both receptors at once.”
Funding: This research was supported by the Russian Science Foundation.
Source: Ilyana Zolotareva – Moscow Institute of Physics and Technology
Publisher: Organized by NeuroscienceNews.com.
Image Source: NeuroscienceNews.com image is credited to Xiaoting Li et al./Cell.
Original Research: Abstract for “Crystal Structure of the Human Cannabinoid Receptor CB2” by Xiaoting Li, Tian Hua, Kiran Vemuri, Jo-Hao Ho, Yiran Wu, Lijie Wu, Petr Popov, Othman Benchama, Nikolai Zvonok, K’ara Locke, Lu Qu, Gye Won Han, Malliga R. Iyer, Resat Cinar, Nathan J. Coffey, Jingjing Wang, Meng Wu, Vsevolod Katritch, Suwen Zhao, George Kunos, Laura M. Bohn, Alexandros Makriyannis, Raymond C. Stevens, and Zhi-Jie Liu in Cell. Published January 10 2019.
doi:10.1016/j.cell.2018.12.011
[cbtabs][cbtab title=”MLA”]Moscow Institute of Physics and Technology “Study Reveals Structure of the Second Human Cannabinoid Receptor.” NeuroscienceNews. NeuroscienceNews, 27 February 2019.
<https://neurosciencenews.com/cannabinoid-receptor-structure-10827/>.[/cbtab][cbtab title=”APA”]Moscow Institute of Physics and Technology (2019, February 27). Study Reveals Structure of the Second Human Cannabinoid Receptor. NeuroscienceNews. Retrieved February 27, 2019 from https://neurosciencenews.com/cannabinoid-receptor-structure-10827/[/cbtab][cbtab title=”Chicago”]Moscow Institute of Physics and Technology “Study Reveals Structure of the Second Human Cannabinoid Receptor.” https://neurosciencenews.com/cannabinoid-receptor-structure-10827/ (accessed February 27, 2019).[/cbtab][/cbtabs]
Abstract
Crystal Structure of the Human Cannabinoid Receptor CB2
The cannabinoid receptor CB2 is predominately expressed in the immune system, and selective modulation of CB2 without the psychoactivity of CB1 has therapeutic potential in inflammatory, fibrotic, and neurodegenerative diseases. Here, we report the crystal structure of human CB2 in complex with a rationally designed antagonist, AM10257, at 2.8 Å resolution. The CB2-AM10257 structure reveals a distinctly different binding pose compared with CB1. However, the extracellular portion of the antagonist-bound CB2 shares a high degree of conformational similarity with the agonist-bound CB1, which led to the discovery of AM10257’s unexpected opposing functional profile of CB2 antagonism versus CB1 agonism. Further structural analysis using mutagenesis studies and molecular docking revealed the molecular basis of their function and selectivity for CB2 and CB1. Additional analyses of our designed antagonist and agonist pairs provide important insight into the activation mechanism of CB2. The present findings should facilitate rational drug design toward precise modulation of the endocannabinoid system.






