Summary: Researchers report an antibiotic usually administered prior to surgery to prevent infection could help reduce damage to the brain that occurs during the early stages of Alzheimer’s disease.
Source: University of British Columbia.
New research from the Djavad Mowafaghian Centre for Brain Health at UBC has found a way to partially restore brain cell communication around areas damaged by plaques associated with Alzheimer’s disease.
The findings, published this week in Nature Communications, demonstrate a possible target and a potential drug treatment to reduce damage to the brain that occurs in the early stages of Alzheimer’s disease. Using Ceftriaxone, an FDA-approved antibiotic used to treat bacterial infections, researchers were able to reduce synaptic disruption and clear the lines of neuronal communication in mice.
Amyloid plaques of -amyloid deposits develop in brain regions of patients with Alzheimer’s disease, These plaques are linked to the damage found in Alzheimer’s disease because they prevent cell communication and are toxic to nerve cells. The researchers found that the brain areas around these plaques show high levels of glutamate, a signaling molecule essential to communication between brain cells, accompanying high levels of hyperactivity in glia, the brain’s support cells. It’s in this glutamate-rich environment that communication between neurons is changed or disrupted, causing neurons to die in the later stages of the disease.
“By imaging the glial cells and glutamate itself around the plaques, we were able to see that the cells were not able to ‘remove’ the glutamate accumulating in these brain areas. By using Ceftriaxone, we were able to up-regulate glutamate transport,” explains Dr. MacVicar, principal investigator and professor of psychiatry. “By restoring glutamate levels, we were able to mostly restore neuronal activity.”
The team’s findings have implications for treatment of early symptoms of Alzheimer’s disease.
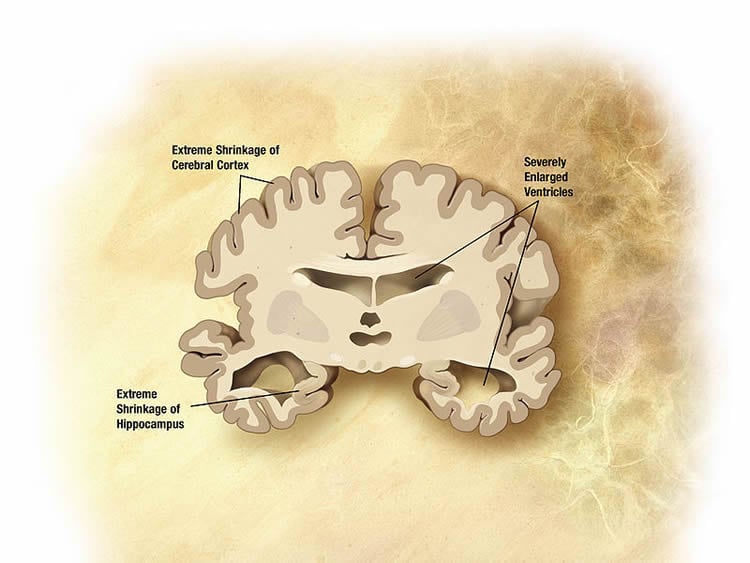
“This dysfunction in cell communication occurs at a very early stage in the disease, before memory impairment is detectable,” says Dr. Jasmin Hefendehl, a former Postdoctoral Fellow in Dr. MacVicar’s lab and the lead author on the paper. “This makes our discovery particularly interesting, as it opens a window for an early intervention strategy to possibly prevent or delay neuron and memory loss.”
Ceftriaxone is an antibiotic that is commonly administered before some types of surgery to prevent infections. Although a recent clinical trial failed to see improvements for treating amyotrophic lateral sclerosis (ALS), the researchers are hopeful about its potential for early intervention in treating Alzheimer’s disease.
Funding: This study was funded by the University of British Columbia Foundation. Dr. Fang, a visiting associate professor of neurology, was supported by the China Scholarship Council Exchange. The authors and University of British Columbia in general disclose conflicts of interest related to this research in the published paper.
Source: Emily Wight – University of British Columbia
Image Source: NeuroscienceNews.com image is in the public domain.
Original Research: Full open access research for “Mapping synaptic glutamate transporter dysfunction in vivo to regions surrounding Aβ plaques by iGluSnFR two-photon imaging” by J. K. Hefendehl, J. LeDue, R. W. Y. Ko, J. Mahler, T. H. Murphy and B. A. MacVicar in Nature Communications. Published online October 7 2016 doi:10.1038/ncomms13441
[cbtabs][cbtab title=”MLA”]University of British Columbia. “Antibiotic Restores Brain Cell Communication in Areas Damaged by Alzheimer’s.” NeuroscienceNews. NeuroscienceNews, 15 November 2016.
<https://neurosciencenews.com/ceftriaxone-antibiotic-alzheimers-5518/>.[/cbtab][cbtab title=”APA”]University of British Columbia. (2016, November 15). Antibiotic Restores Brain Cell Communication in Areas Damaged by Alzheimer’s. NeuroscienceNews. Retrieved November 15, 2016 from https://neurosciencenews.com/ceftriaxone-antibiotic-alzheimers-5518/[/cbtab][cbtab title=”Chicago”]University of British Columbia. “Antibiotic Restores Brain Cell Communication in Areas Damaged by Alzheimer’s.” https://neurosciencenews.com/ceftriaxone-antibiotic-alzheimers-5518/ (accessed November 15, 2016).[/cbtab][/cbtabs]
Abstract
Mapping synaptic glutamate transporter dysfunction in vivo to regions surrounding Aβ plaques by iGluSnFR two-photon imaging
Amyloid-β (Aβ) plaques, a hallmark of Alzheimer’s disease (AD), are surrounded by regions of neuronal and glial hyperactivity. We use in vivo two-photon and wide-field imaging of the glutamate sensor iGluSnFR to determine whether pathological changes in glutamate dynamics in the immediate vicinity of Aβ deposits in APPPS1 transgenic mice could alter neuronal activity in this microenvironment. In regions close to Aβ plaques chronic states of high spontaneous glutamate fluctuations are observed and the timing of glutamate responses evoked by sensory stimulation exhibit slower decay rates in two cortical brain areas. GLT-1 expression is reduced around Aβ plaques and upregulation of GLT-1 expression and activity by ceftriaxone partially restores glutamate dynamics to values in control regions. We conclude that the toxic microenvironment surrounding Aβ plaques results, at least partially, from enhanced glutamate levels and that pharmacologically increasing GLT-1 expression and activity may be a new target for early therapeutic intervention.
“Mapping synaptic glutamate transporter dysfunction in vivo to regions surrounding Aβ plaques by iGluSnFR two-photon imaging” by J. K. Hefendehl, J. LeDue, R. W. Y. Ko, J. Mahler, T. H. Murphy and B. A. MacVicar in Nature Communications. Published online October 7 2016 doi:10.1038/ncomms13441






