Summary: A new study reveals both genetics and environment play a role in shaping brain connectivity.
Source: SfN.
A new mechanism regulating the early development of connections between the two sides of the nervous system has been identified in a paper published in eNeuro. The work demonstrates that neuronal activity is required for this process, a finding that may provide new insight into brain connectivity disorders such as autism.
The paths of axons in the central nervous system are laid down during embryonic development. Most of them will cross the middle of the organism, while some will not. The resulting framework is critical for the connectivity that will later give rise to cognitive functions. Although axon pathfinding is under tight genetic control, the extent to which it can be influenced by environmental factors is unclear.
Addressing this question in transparent zebrafish embryos, Josh Bonkowsky and colleagues found that optogenetic stimulation of an inhibited N-methyl-D-aspartate receptor (NMDAR) was necessary for axons to properly cross the midline. Regulation of neuronal activity by the NMDAR may act through a gene implicated in brain development and neurological diseases.
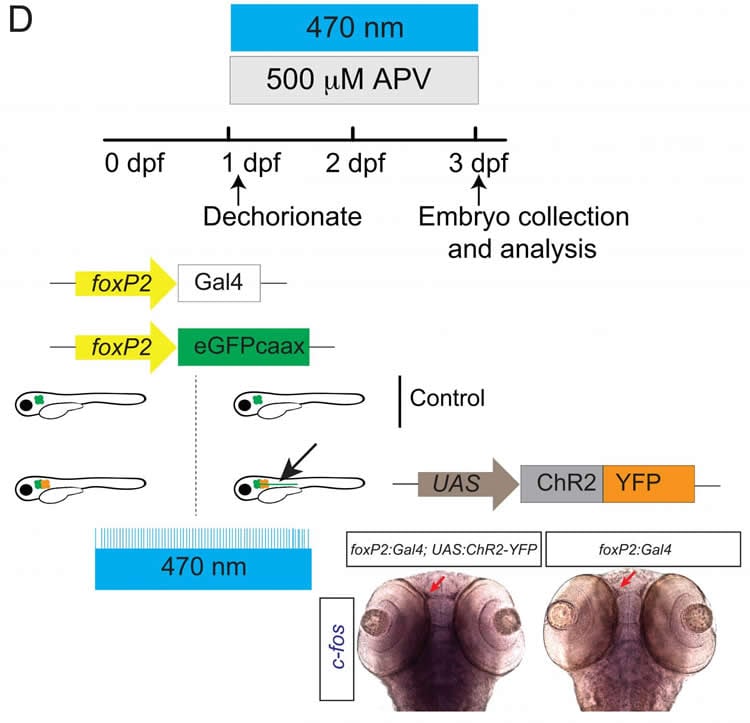
This research suggests that, in addition to genetic control, neuronal activity in response to environmental factors — for example, the low oxygen levels experienced by premature babies — can influence the development of brain connectivity.
Funding: The work was funded by the National Institutes of Health, NIH/National Institute of Mental Health.
Source: David Barnstone – SfN
Publisher: Organized by NeuroscienceNews.com.
Image Source: NeuroscienceNews.com image is credited to Gao et al., eNeuro (2018).
Original Research: Abstract for “The Midline Axon Crossing Decision Is Regulated through an Activity-Dependent Mechanism by the NMDA Receptor” by Jingxia Gao, Tamara J. Stevenson, Adam D. Douglass, Josh Barrios and Joshua Bonkowsky in eNeuro. Published April 16 2018.
doi:10.1523/ENEURO.0389-17.2018
[cbtabs][cbtab title=”MLA”]SfN “Early Environment May Shape Axon Pathfinding.” NeuroscienceNews. NeuroscienceNews, 16 April 2018.
<https://neurosciencenews.com/axon-pathfinding-8814/>.[/cbtab][cbtab title=”APA”]SfN (2018, April 16). Early Environment May Shape Axon Pathfinding. NeuroscienceNews. Retrieved April 16, 2018 from https://neurosciencenews.com/axon-pathfinding-8814/[/cbtab][cbtab title=”Chicago”]SfN “Early Environment May Shape Axon Pathfinding.” https://neurosciencenews.com/axon-pathfinding-8814/ (accessed April 16, 2018).[/cbtab][/cbtabs]
Abstract
The Midline Axon Crossing Decision Is Regulated through an Activity-Dependent Mechanism by the NMDA Receptor
Axon guidance in vertebrates is controlled by genetic cascades as well as by intrinsic activity-dependent refinement of connections. Midline axon crossing is one of the best studied pathfinding models and is fundamental to the establishment of bilaterally symmetric nervous systems. However, it is not known whether crossing requires intrinsic activity in axons, and what controls that activity. Further, a mechanism linking neuronal activity and gene expression has not been identified for axon pathfinding. Using embryonic zebrafish, we found that the N-methyl-D-aspartate (NMDA) receptor (NMDAR) NR1.1 subunit (grin1a) is expressed in commissural axons. Pharmacological inhibition of grin1a, hypoxia exposure reduction of grin1a expression, or CRISPR knockdown of grin1a, leads to defects in midline crossing. Inhibition of neuronal activity phenocopies the effects of grin1a loss on midline crossing. By combining pharmacological inhibition of the NMDAR with optogenetic stimulation to precisely restore neuronal activity, we observed rescue of midline crossing. This suggests that the NMDAR controls pathfinding by an activity-dependent mechanism. We further show that the NMDAR may act, via modulating activity, on the transcription factor arxa (mammalian Arx), a known regulator of midline pathfinding. These findings uncover a novel role for the NMDAR in controlling activity to regulate commissural pathfinding, and identify arxa as a key link between the genetic and activity-dependent regulation of midline axon guidance.
Significance Statement While intrinsic neuronal activity is involved in refinement of axon connections, its role in pathfinding decisions is poorly understood. We found that midline axon crossing is regulated by the NMDA receptor (NMDAR). The NMDAR is expressed on axons that cross the midline, and inhibition or knockdown of the NDMDAR led to fewer axons crossing. Precise optogenetic stimulation of neurons rescued the effects of NMDAR blockade, demonstrating that the NMDAR acts by an activity-dependent mechanism. In turn, the NMDAR affects expression of arxa, an important gene for brain development and that is associated with several human neurologic diseases. These results show a critical role for the NMDAR in early axon guidance decisions by control of neuronal activity.






