Mutated protein weakens cytoskeletal structure.
Using an innovative exome sequencing strategy, a team of international scientists led by John Landers, PhD, at the University of Massachusetts Medical School has shown that TUBA4A, the gene encoding the Tubulin Alpha 4A protein, is associated with familial amyotrophic lateral sclerosis (ALS), a fatal neurological disorder also known as Lou Gehrig’s Disease. Details of the study were published today in Neuron.
Exome sequencing, in contrast to whole genome sequencing, relies on sequencing only the protein-coding genes in a genome and has been an effective and cost-efficient strategy for identifying disease-causing genetic mutations. However, exome sequencing can be limited in its application because all people harbor many rare genetic variants that don’t cause disease and are nothing more than normal genetic background noise. Isolating disease-causing genetic variants requires DNA from multiple people in an affected family to be successful. For late-onset diseases such as ALS, finding enough patients to sequence in a single family is not always possible.
To overcome this limitation, Landers and colleagues performed an exome-wide screen on 363 people with familial ALS, each of whom also had a family member with the condition. Researchers performed an analysis of every coding gene in the genome of these patients and then they searched for patterns of rare, damaging mutations that appeared more frequently in patients with ALS than in the general population.
“Every single one of us carries rare mutations which make the identification of disease-associated genes difficult. By analyzing the mutation rate of every gene in our patients and comparing them to the general population, we were able to show that the TUBA4A gene had an elevated frequency of mutation in patients,” said Landers, a professor of neurology at UMMS.
Additional testing by Landers and colleagues found that mutation to the TUBA4A gene was, in fact, associated with ALS.
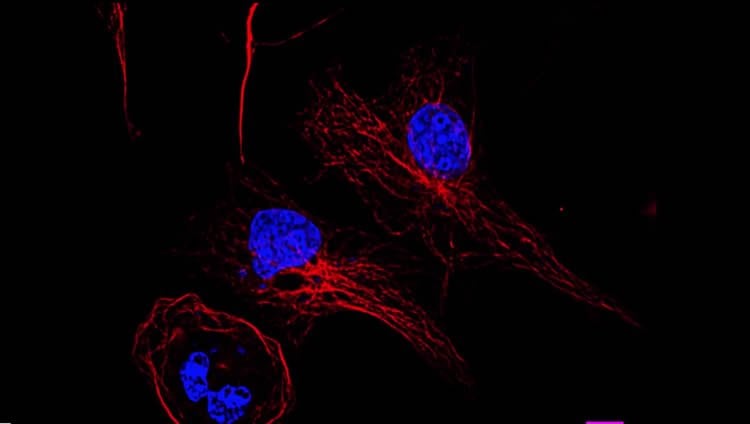
TUBA4A encodes for the Tubulin Alpha 4A protein and is found in all human tissue with its highest levels of expression in the brain. In nerve cells, TUBA4A is responsible for moving vital building blocks from one part of the cell to another. Specifically, TUBA4A helps build the microtubule network, one of the most important structural components of the nerve cell. Landers and colleagues found that the mutated TUBA4A protein is toxic to the neuron by weakening the entire microtubule network.
“This demonstrates the importance of identifying even rare mutations that could cause ALS, as they might point to common pathways that are altered leading to motor neuron death,” said Landers. “TUBA4A is one of several cytoskeletal genes that are associated with ALS. This strengthens the thought that the cytoskeleton architecture and dynamics may play a major role in the development of the disease.”
This video describes the identification of TUBA4A, encoding the Tubulin, Alpha 4A protein, as a novel gene associated with familial ALS through the exome sequencing of hundreds of patients. Functional analyses revealed that TUBA4A mutants lead to a destabilized microtubule network and diminished.
ALS is a progressive, neurodegenerative disorder affecting the motor neurons in the central nervous system. As motor neurons die, the brain’s ability to send signals to the body’s muscles is compromised. This leads to loss of voluntary muscle movement, paralysis and eventually respiratory failure. The cause of most cases of ALS is not known. Approximately 10 percent of cases are inherited. Though investigators at UMMS and elsewhere have identified several genes shown to cause inherited or familial ALS, nearly one-third of these cases have an unknown genetic cause.
“The ongoing efforts from the ALS research community to sequence thousands of patient genomes through large international collaborations such as this will be an invaluable resource to identifying new disease-causing genes and pathways that could potentially be targeted for therapeutic intervention,” said Landers.
Contact: Jim Fessenden – University of Massachusetts Medical School
Source: University of Massachusetts Medical School press release
Image Source: The image is adapted from the cellvideoabstracts video and is available from the University of Massachusetts Medical School press release
Video Source: The video “TUBA4A Mutations Are Associated with Familial ALS” is available at the cellvideoabstracts YouTube page
Original Research: Abstract for “Exome-wide Rare Variant Analysis Identifies TUBA4A Mutations Associated with Familial ALS” by Bradley N. Smith, Nicola Ticozzi, Claudia Fallini, Athina Soragia Gkazi, Simon Topp, Kevin P. Kenna, Emma L. Scotter, Jason Kost, Pamela Keagle, Jack W. Miller, Daniela Calini, Caroline Vance, Eric W. Danielson, Claire Troakes, Cinzia Tiloca, Safa Al-Sarraj, Elizabeth A. Lewis, Andrew King, Claudia Colombrita, Viviana Pensato, Barbara Castellotti, Jacqueline de Belleroche, Frank Baas, Anneloor LMA ten Asbroek, Peter C. Sapp, Diane McKenna-Yasek, Russell L. McLaughlin, Meraida Polak, Seneshaw Asress, Jesús Esteban-Pérez, José Luis Muñoz-Blanco, Michael Simpson, SLAGEN Consortium, Wouter van Rheenen, Frank P. Diekstra, Giuseppe Lauria, Stefano Duga, Stefania Corti, Cristina Cereda, Lucia Corrado, Gianni Sorarù, Karen E. Morrison, Kelly L. Williams, Garth A. Nicholson, Ian P. Blair, Patrick A. Dion, Claire S. Leblond, Guy A. Rouleau, Orla Hardiman, Jan H. Veldink, Leonard H. van den Berg, Ammar Al-Chalabi, Hardev Pall, Pamela J. Shaw, Martin R. Turner, Kevin Talbot, Franco Taroni, Alberto García-Redondo, Zheyang Wu, Jonathan D. Glass, Cinzia Gellera, Antonia Ratti, Robert H. Brown Jr., Vincenzo Silani, Christopher E. Shaw, John E. Landers in Neuron. Published online October 22 2014 doi:10.1016/j.neuron.2014.09.027






