A multicenter study led by Christian Haass and Michael Ewers of Ludwig-Maximilians-Universitaet (LMU) in Munich has identified a biomarker associated with the activation of an innate immune response to neural damage during early stages of Alzheimer’s disease.
Alzheimer’s disease results from the accumulation in the brain of protein deposits that are toxic to nerve cells. The deposits themselves are largely comprised of insoluble aggregates of so-called beta-amyloid peptides – short fragments cleaved from a protein found on nerve-cell membranes – and they begin to form decades before any overt symptoms of dementia emerge. Now research teams led by Christian Haass, who holds the Chair of Metabolic Biochemistry at LMU and is the speaker of the German Center for Neurodegenerative Diseases (DZNE) in Munich, and Professor Michael Ewers of the Institute for Stroke and Dementia Research (ISD) at Munich University Medical Center, now report that the concentration of a specific segment of the protein TREM2 in cerebrospinal fluid (CSF) is significantly elevated in early stages of Alzheimer’s disease. “Our findings indicate that TREM2 plays an important role in the progression of Alzheimer’s, and perhaps even other forms of dementia. It appears to be part of a defense mechanism that involves phagocytic cells that eliminate damaged nerve cells and toxic protein deposits, such as those made up of beta-amyloid peptides,” says Haass. The new study has now been published in the journal EMBO Molecular Medicine.
TREM2 is a cell-surface receptor that is essential for the function of specialized phagocytic cells called microglia, which are found only in the brain. Microglial cells serve as an arm of the innate immune system and act as the brain’s waste disposal squad, recognizing and destroying cell debris and other types of toxic particulate material. The detection by Haass, Ewers and colleagues of increased concentrations of a soluble fragment of TREM2 in the CSF of patients with mild symptoms of Alzheimer’s disease forges a further link between the protein and the neurodegenerative disorder. The same researchers had previously shown that mutations that affect TREM2 function also reduce the ability of microglia to clear amyloid aggregates and damaged cells.
An informative and versatile marker
In their latest work, the LMU team studied over 400 Alzheimer’s patients who showed cognitive defects of varying severity, and compared them with age-matched controls. Biochemical analyses of samples of CSF revealed that patients who exhibited mild cognitive deficits had the highest concentrations of a particular fragment of the TREM2 protein found in the study. The amounts detected in patients with advanced Alzheimer’s disease were significantly lower. “This correlates with the level of activity of the microglial cells, which falls off as the condition progresses. This in turn presumably means that less beta-amyloid and cell debris can be cleared away,” Haass points out. “We therefore believe that this biomarker provides us with a way to assess the capacity of innate immune cells in the brain to degrade and dispose toxic material.”
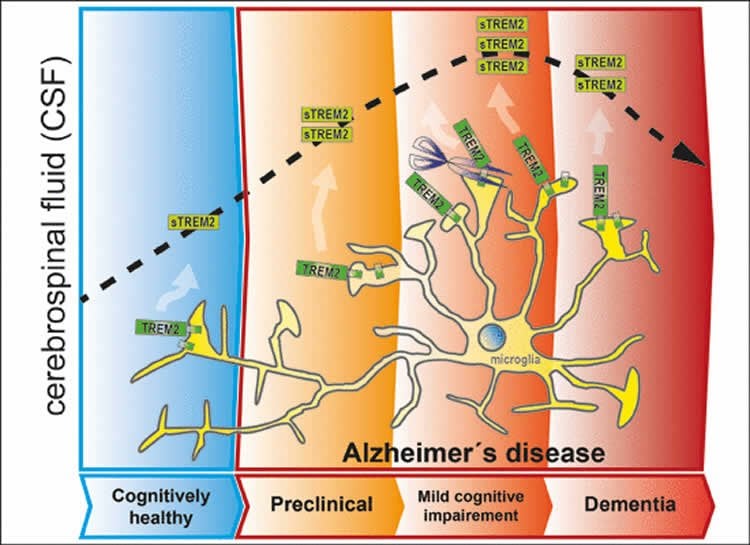
At the current stage it is still unclear whether the changes in the concentration of the TREM2 fragment in the CSF is a cause or a consequence of disease progression. However, the researchers favor the idea that the early increase in the level of TREM2 is attributable to the activation of microglia in response to initial signs of damage to the nerve cells in the brain. “Our results indicate that the alterations in TREM2 reflect physiological changes that occur at an early stage in the development of Alzheimer’s dementia. This makes TREM2 interesting from a therapeutic perspective,” says Michael Ewers.
The new biomarker could also make it possible to monitor the efficacy of novel anti-inflammatory approaches for treatment of Alzheimer’s disease. In addition, measurements of the levels of TREM2 in the CSF could help to define the most effective window for early interventions to combat or control the disorder. The LMU researchers propose a long-term longitudinal study, in which the concentration of TREM2 in CSF samples from individuals with mutations known to be linked to familial Alzheimer’s disease is determined at regular intervals under controlled conditions.
Source: Luise Discherl – LMUM
Image Credit: The image is credited to The researchers/EMBO Journals.
Original Research: Full open access research for “sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early‐stage Alzheimer’s disease and associate with neuronal injury markers” by Marc Suárez‐Calvet, Gernot Kleinberger, Miguel Ángel Araque Caballero, Matthias Brendel, Axel Rominger, Daniel Alcolea, Juan Fortea, Alberto Lleó, Rafael Blesa, Juan Domingo Gispert, Raquel Sánchez‐Valle, Anna Antonell, Lorena Rami, José L Molinuevo, Frederic Brosseron, Andreas Traschütz, Michael T Heneka, Hanne Struyfs, Sebastiaan Engelborghs, Kristel Sleegers, Christine Van Broeckhoven, Henrik Zetterberg, Bengt Nellgård, Kaj Blennow, Alexander Crispin, Michael Ewers, and Christian Haass in EMBO Molecular Medicine. Published online March 3 2016 doi:10.15252/emmm.201506123
Abstract
sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early‐stage Alzheimer’s disease and associate with neuronal injury markers
TREM2 is an innate immune receptor expressed on the surface of microglia. Loss‐of‐function mutations of TREM2 are associated with increased risk of Alzheimer’s disease (AD). TREM2 is a type‐1 protein with an ectodomain that is proteolytically cleaved and released into the extracellular space as a soluble variant (sTREM2), which can be measured in the cerebrospinal fluid (CSF). In this cross‐sectional multicenter study, we investigated whether CSF levels of sTREM2 are changed during the clinical course of AD, and in cognitively normal individuals with suspected non‐AD pathology (SNAP). CSF sTREM2 levels were higher in mild cognitive impairment due to AD than in all other AD groups and controls. SNAP individuals also had significantly increased CSF sTREM2 compared to controls. Moreover, increased CSF sTREM2 levels were associated with higher CSF total tau and phospho‐tau181P, which are markers of neuronal degeneration and tau pathology. Our data demonstrate that CSF sTREM2 levels are increased in the early symptomatic phase of AD, probably reflecting a corresponding change of the microglia activation status in response to neuronal degeneration.
“sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early‐stage Alzheimer’s disease and associate with neuronal injury markers” by Marc Suárez‐Calvet, Gernot Kleinberger, Miguel Ángel Araque Caballero, Matthias Brendel, Axel Rominger, Daniel Alcolea, Juan Fortea, Alberto Lleó, Rafael Blesa, Juan Domingo Gispert, Raquel Sánchez‐Valle, Anna Antonell, Lorena Rami, José L Molinuevo, Frederic Brosseron, Andreas Traschütz, Michael T Heneka, Hanne Struyfs, Sebastiaan Engelborghs, Kristel Sleegers, Christine Van Broeckhoven, Henrik Zetterberg, Bengt Nellgård, Kaj Blennow, Alexander Crispin, Michael Ewers, and Christian Haass in EMBO Molecular Medicine. Published online March 3 2016 doi:10.15252/emmm.201506123






