Summary: By inhibiting a particular receptor microglia use to survive, researchers reduced neuroinflammation and prompted recovery following traumatic brain injury in mouse models.
Source: TCD
Targeting overactive immune cells and dampening their chronic neurotoxic effects may offer new therapeutic strategies for traumatic brain injury (TBI), according to new preclinical research in mice, which has been published today in the Journal of Neuroscience.
The collaborative research, which involved scientists from Trinity College Dublin and the University of Maryland School of Medicine, Baltimore, USA, also suggests that the impact of TBI on brain degeneration may be modifiable a relatively long time after the injury was sustained – something at odds with current thinking.
Traumatic brain injury and delayed treatment
Triggered by trauma, microglia – the brain’s immune cells – morph into an inflammatory state, which helps to protect the brain. However, long-term inflammation after TBI may contribute to neurological degeneration and cognitive declines similar to those observed in TBI-associated neurodegenerative diseases, such as chronic traumatic encephalopathy and Alzheimer’s disease.
The scientists involved in the new study found that highly delayed targeting of chronic inflammation pathways may be a very effective therapeutic strategy for TBI.
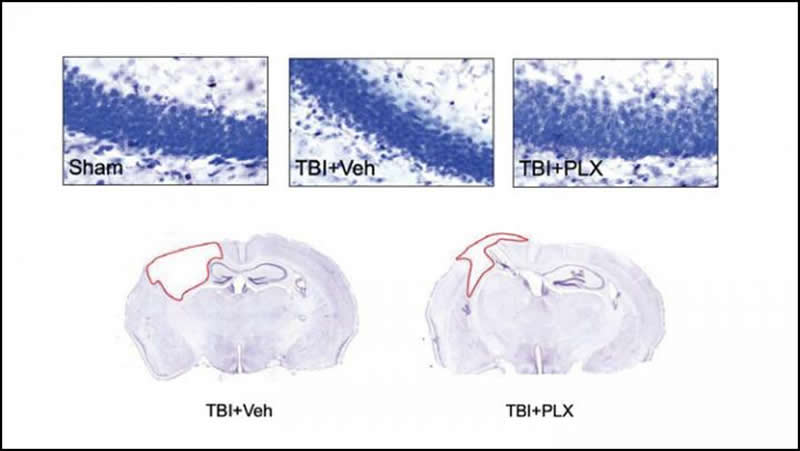
One month after a TBI, the team inhibited a particular receptor microglia need to survive. The inhibition killed 95% of the mice microglia cells. However, the cells bounced back to normal levels once the inhibition ends.
The researchers then stopped the inhibition after one week and let the mice recover for a few months. They found that the inhibition essentially “reset” the mice’s microglia: the new cells were in a more normal, less inflammatory state. The mice recovered better than the mice that didn’t receive treatment, showing less brain damage, fewer neuron deaths, and better motor and cognitive performance.
David Loane, Research assistant professor in Trinity’s School of Biochemistry and Immunology, said:
“These preclinical studies suggest that the consequences of TBI on brain degeneration and related neurological impairment may be modifiable quite a long time after injury by targeting inflammation pathways, which is a finding at odds with widely accepted views about head injury.”
“The exciting thing is the possibility that we may one day be able to minimise brain degeneration and neurological impairment in people who have suffered a TBI. It will of course always be incredibly important to act quickly whenever someone suffers a TBI, but our findings suggest targeting inflammation pathways further down the treatment line may make a major difference to long-term brain health and recovery.”
Dr Loane has recently established a state-of-the-art, preclinical research laboratory in Trinity to study the neuroimmunology of TBI and related dementias. His work is supported by an SFI President of Ireland Future Research Leaders Award and the National Institutes of Health (USA).
Source:
TCD
Media Contacts:
David Loane – TCD
Image Source:
The image is Henry et al., JNeurosci 2020.
Original Research: Closed access
“Microglial depletion with CSF1R inhibitor during chronic phase of experimental traumatic brain injury reduces neurodegeneration and neurological deficits”. Rebecca J. Henry, Rodney M. Ritzel, James P. Barrett, Sarah J. Doran, Yun Jiao, Jennie B. Leach, Gregory L. Szeto, Junfang Wu, Bogdan A. Stoica, Alan I. Faden and David J. Loane.
Journal of Neuroscience doi:10.1523/JNEUROSCI.2402-19.2020.
Abstract
Microglial depletion with CSF1R inhibitor during chronic phase of experimental traumatic brain injury reduces neurodegeneration and neurological deficits
Chronic neuroinflammation with sustained microglial activation occurs following severe traumatic brain injury (TBI) and is believed to contribute to subsequent neurodegeneration and neurological deficits . Microglia, the primary innate immune cells in brain, are dependent on colony stimulating factor 1 receptor (CSF1R) signaling for their survival. In this pre-clinical study, we examined the effects of delayed depletion of chronically activated microglia on functional recovery and neurodegeneration up to three months post-injury. A CSF1R inhibitor, PLX5622, was administered to adult male C57Bl/6J mice at one month after controlled cortical impact to remove chronically activated microglia, and the inhibitor was withdrawn 1-week later to allow for microglial repopulation. Following TBI, the repopulated microglia displayed a ramified morphology similar to that of sham uninjured mice, whereas microglia in vehicle-treated TBI mice showed the typical chronic posttraumatic hypertrophic morphology. PLX5622 treatment limited TBI-associated neuropathological changes at 3 months post-injury; these included a smaller cortical lesion, reduced hippocampal neuron cell death, and decreased NOX2- and NLRP3 inflammasome-associated neuroinflammation. Furthermore, delayed depletion of chronically activated microglia after TBI led to widespread changes in the cortical transcriptome and altered gene pathways involved in neuroinflammation, oxidative stress, and neuroplasticity. Using a variety of complementary neurobehavioral tests, PLX5622-treated TBI mice also had improved long-term motor and cognitive function recovery through 3 months post-injury. Together, these studies demonstrate that chronic phase removal of neurotoxic microglia after TBI using CSF1R inhibitors markedly reduce chronic neuroinflammation and associated neurodegeneration, as well as related motor and cognitive deficits.
SIGNIFICANCE STATEMENT
Traumatic brain injury (TBI) is a debilitating neurological disorder that can seriously impact the patient’s quality of life. Microglial-mediated neuroinflammation is induced after severe TBI and contributes to neurological deficits and on-going neurodegenerative processes. Here, we investigated the effect of breaking the neurotoxic neuroinflammatory loop at 1-month after controlled cortical impact in mice by pharmacological removal of chronically activated microglia using a CSF1R inhibitor, PLX5622. Overall, we show that short-term elimination of microglia during the chronic phase of TBI followed by repopulation results in long-term improvements in neurological function, suppression of neuroinflammatory and oxidative stress pathways, and a reduction in persistent neurodegenerative processes. These studies are clinically relevant and support new concepts that the therapeutic window for TBI may be far longer than traditionally believed if chronic and evolving microglial-mediated neuroinflammation can be inhibited or regulated in a precise manner.






