Summary: Researchers have discovered a mechanism of glucose sensing by muscles that contribute to the regulation of blood sugar levels in the body.
Source: University of Michigan.
It’s obvious that the taste buds on the tongue can detect sugar. And after a meal, beta cells in the pancreas sense rising blood glucose and release the hormone insulin—which helps the sugar enter cells, where it can be used by the body for energy.
Now researchers at the University of Michigan Life Sciences Institute have uncovered an unexpected mechanism of glucose sensing in skeletal muscles that contributes to the body’s overall regulation of blood sugar levels.
“We found that skeletal muscle cells have machinery to directly sense glucose—in a certain sense it’s like the muscles can taste sugar, too,” said senior study author Jiandie Lin, a faculty member at the LSI, where his lab is located.
This ability of muscles to sense blood glucose is a separate and parallel process that augments the insulin-driven response. Together they work as a rheostat to maintain steady glucose levels in the body, particularly after a meal, according to findings published May 4 in Molecular Cell.
Continuing to develop this in-depth understanding of how the body self-regulates blood sugar at the molecular level could shed new light on obesity and diabetes, as well as point toward new therapeutic targets, said Zhuoxian Meng, the study’s lead author and a research investigator in Lin’s lab.
The researchers were able to examine the contributions of the glucose-sensing pathway in skeletal muscle by silencing a key gene—BAF60C—in cell cultures and in laboratory mice.
“When we did that, the mice lacking BAF60C looked absolutely normal, but after we gave them a high-fat diet to induce obesity, they developed trouble disposing of the additional glucose after a meal,” Lin said. “The well-known insulin mechanism was not sufficient to process the glucose on its own.”
Elevated blood sugar following a meal is a key symptom of Type 2 diabetes. And chronic high blood sugar, also known hyperglycemia, can lead to serious health issues.
“We found that the molecular pathway that’s engaged by glucose in muscle cells, at least the initial steps, is very similar to what happens in the beta cells in the pancreas,” said Lin, who is also a professor of cell and developmental biology at the U-M Medical School. “This is very interesting because there’s a very important class of diabetes drugs known as sulfonylureas that act by closing a potassium channel and causing the beta cells to secrete more insulin.
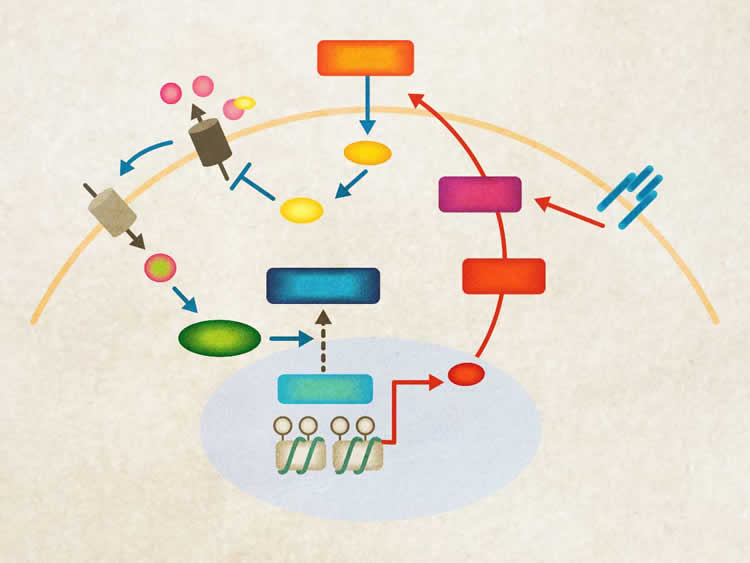
“Our research shows that this glucose-sensing pathway in muscle cells likely also plays a role in the drugs’ overall glucose-lowering action. The extent of the pathway’s contribution will need to be studied further.”
Additionally, Lin said, there are two steps within the glucose-sensing pathway that could serve as potential targets for modulation with therapeutic compounds.
“It’s amazing how subtle changes in glucose can be detected throughout the body,” Lin said. “Beta cells respond, nerve cells respond, and now we know that muscle cells respond directly, too.”
Funding: The research was supported by grants from the National Institutes of Health and the American Heart Association.
Additional authors include: Zhimin Chen, Jingxia Sun, Yuanyuan Xiao, Lin Wang, Yaqiang Li and Shawn Xu from U-M; Jianfeng Liu from Huazhong University of Science and Technology, China; and Jianke Gong from U-M and Huazhong University of Science and Technology.
Source: Stephanie King – University of Michigan
Image Source: NeuroscienceNews.com image is credited to Stephanie King/LSI.
Original Research: Abstract for “Glucose Sensing by Skeletal Myocytes Couples Nutrient Signaling to Systemic Homeostasis” by Zhuo-Xian Meng, Jianke Gong, Zhimin Chen, Jingxia Sun, Yuanyuan Xiao, Lin Wang, Yaqiang Li, Jianfeng Liu, X. Z. Shawn Xu, and Jiandie D. Lin in Molecular Cell. Published online April 5 2017 doi:10.1016/j.molcel.2017.04.007
[cbtabs][cbtab title=”MLA”]University of Michigan “Your Muscles Can ‘Taste’ Sugar.” NeuroscienceNews. NeuroscienceNews, 5 May 2017.
<https://neurosciencenews.com/sugar-muscles-6596/>.[/cbtab][cbtab title=”APA”]University of Michigan (2017, May 5). Your Muscles Can ‘Taste’ Sugar. NeuroscienceNew. Retrieved May 5, 2017 from https://neurosciencenews.com/sugar-muscles-6596/[/cbtab][cbtab title=”Chicago”]University of Michigan “Your Muscles Can ‘Taste’ Sugar.” https://neurosciencenews.com/sugar-muscles-6596/ (accessed May 5, 2017).[/cbtab][/cbtabs]
Abstract
Glucose Sensing by Skeletal Myocytes Couples Nutrient Signaling to Systemic Homeostasis
Highlights
•Skeletal myocytes engage in physiological glucose sensing via the KATP channel
•Glucose stimulates insulin-independent AKT activation through HDAC5/Baf60c
•Muscle glucose sensing is required for postprandial glucose homeostasis
•Sulfonylureas lower blood glucose in part through the Baf60c-Deptor-AKT axis
Summary
Skeletal muscle is a major site of postprandial glucose disposal. Inadequate insulin action in skeletal myocytes contributes to hyperglycemia in diabetes. Although glucose is known to stimulate insulin secretion by β cells, whether it directly engages nutrient signaling pathways in skeletal muscle to maintain systemic glucose homeostasis remains largely unexplored. Here we identified the Baf60c-Deptor-AKT pathway as a target of muscle glucose sensing that augments insulin action in skeletal myocytes. Genetic activation of this pathway improved postprandial glucose disposal in mice, whereas its muscle-specific ablation impaired insulin action and led to postprandial glucose intolerance. Mechanistically, glucose triggers KATP channel-dependent calcium signaling, which promotes HDAC5 phosphorylation and nuclear exclusion, leading to Baf60c induction and insulin-independent AKT activation. This pathway is engaged by the anti-diabetic sulfonylurea drugs to exert their full glucose-lowering effects. These findings uncover an unexpected mechanism of glucose sensing in skeletal myocytes that contributes to homeostasis and therapeutic action.
“Glucose Sensing by Skeletal Myocytes Couples Nutrient Signaling to Systemic Homeostasis” by Zhuo-Xian Meng, Jianke Gong, Zhimin Chen, Jingxia Sun, Yuanyuan Xiao, Lin Wang, Yaqiang Li, Jianfeng Liu, X. Z. Shawn Xu, and Jiandie D. Lin in Molecular Cell. Published online April 5 2017 doi:10.1016/j.molcel.2017.04.007






