Scientists from Bern have discovered a mechanism which is responsible for the degeneration of Purkinje cells in the cerebellum in a neurodegenerative disease called Spinocerebellar ataxia type 1. The results of their study open up new avenues for the future treatment of cerebellum associated degenerative disorders.
Damage, degeneration or loss of neurons in the region of the brain that controls muscle coordination (cerebellum), results in ataxia. The symptoms include loss of voluntary coordination of muscle movements and the appearance of gait abnormality, loss of balance and speech problems. Cerebellar ataxias are progressive degenerative disorders which occur in adults either sporadically or can be inherited from parents. Unfortunately, the large majority of cerebellar ataxia cases are sporadic in nature and the causative mechanism for the development of ataxia remains largely unknown, which eventually hinders the development of therapy and negatively influences the quality of patient’s life. However, both the sporadic and inherited cases of cerebellar ataxia exhibit common pathophysiological characteristics such as the specific degeneration of the main cerebellar neurons; the Purkinje cells. Therefore, the team of Smita Saxena from the Institute of Cell Biology at the University of Bern set out to understand the potential mechanism involved in the development of ataxia and degeneration of Purkinje cells in Spinocerebellar ataxia type 1 (SCA1), a rare, incurable, inheritable neurodegenerative disease that can be modeled in mice.
Together with first author Céline Ruegsegger, a protein based screening of Purkinje cells was performed to identify changes that occur in these neurons at the time of ataxia appearance. The team discovered wide spread alterations in proteins which function at the synapse and identified a synaptic protein Homer-3 that is mainly present in Purkinje cell synapses to be reduced. Further, they found that Homer-3 decrease was related to the alteration in an important signaling pathway; mTORC1. This signaling pathway was responsible for regulating the expression of synaptic proteins such as Homer-3. Saxena and her team have discovered a cellular mechanism in the cerebellum of SCA1 mice that specifically targets the degeneration of Purkinje cells and the findings present a promising future therapeutic target. The study was published in the scientific journal Neuron.
The team investigated why mTORC1 signaling was altered in the cerebellar Purkinje cells and not in other regions of the brain. By measuring activation state of Purkinje cells, they found that impaired mTORC1 signaling was due to defects in Purkinje cell associated neuronal circuitry mainly involving the climbing fibers. “In this context, the identification of circuit related alterations which play an important role in determining pathological alterations in Purkinje cells is important in understanding how the disease mechanism works and targets vulnerable components in defined neurons; in this case Purkinje cells”, says Saxena.
Reinstating Homer-3 expression can ameliorate symptoms and delay pathology
After the identification of Homer-3 as being reduced early in the disease course, Céline Ruegsegger and Saxena tried to establish its causal role in the development of disease. By using a gene therapy approach they reintroduced Homer-3 expression in Purkinje cells of SCA1 mice. This slowed down the development of ataxia, ameliorated symptoms associated with loss of motor coordination and balance and restored Purkinje cell functionality.
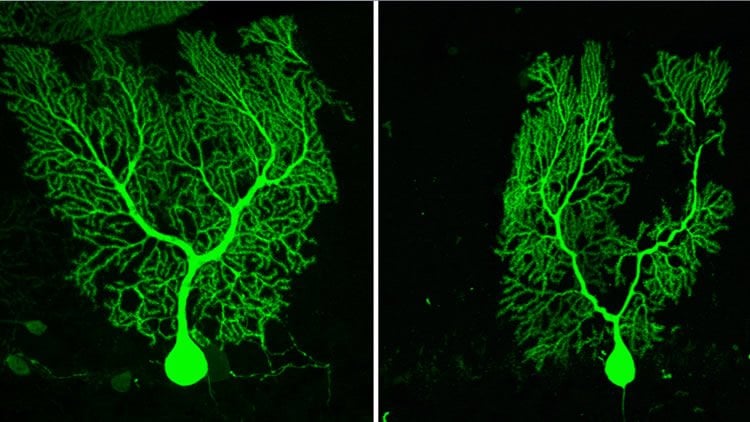
“Interestingly, it has been known for some time that alterations in mTORC1 signaling in the cerebellum during development is associated with autistic behavior and intellectual disorder”, said Saxena. “In our study, the novel finding is that similar signaling pathways can also be involved in adult cerebellar associated degenerative disorders such as SCA1. This is an important step forward in understanding the process involved in developmental and degenerative disorders and identifies a potentially new therapeutic target for the future.”
Source: Smita Saxena – University of Bern
Image Source: The image is credited to Institute of Cell Biology, University of Bern
Original Research: Abstract for “Impaired mTORC1-dependent expression of Homer-3 influences SCA1 pathophysiology” by Céline Ruegsegger, David M. Stucki, Silvio Steiner, Nico Angliker, Julika Radecke, Eva Keller, Benoît Zuber, Markus A. Rüegg and Smita Saxena in SNeuron. Published online January 6 2016 doi:10.1016/j.neuron.2015.11.033
Abstract
Impaired mTORC1-dependent expression of Homer-3 influences SCA1 pathophysiology
Highlights
•Impaired Homer-3 expression and mTORC1 activity in SCA1 Purkinje cells
•mTORC1 signaling regulates Homer-3 expression in cerebellar Purkinje cells
•Purkinje cell-specific mTORC1 deletion worsens SCA1 pathology
•Restoring Homer-3 expression alleviates disease-associated deficits
Summary
Spinocerebellar ataxia type 1 (SCA1), due to the expansion of a polyglutamine repeat within the ubiquitously expressed Ataxin-1 protein, leads to the premature degeneration of Purkinje cells (PCs), the cause of which is poorly understood. Here, we identified the unique proteomic signature of Sca1154Q/2Q PCs at an early stage of disease, highlighting extensive alterations in proteins associated with synaptic functioning, maintenance, and transmission. Focusing on Homer-3, a PC-enriched scaffold protein regulating neuronal activity, revealed an early decline in its expression. Impaired climbing fiber-mediated synaptic transmission diminished mTORC1 signaling, paralleling Homer-3 reduction in Sca1154Q/2Q PCs. Ablating mTORC1 within PCs or pharmacological inhibition of mTORC1 identified Homer-3 as its downstream target. mTORC1 knockout in Sca1154Q/2Q PCs exacerbated and accelerated pathology. Reinstating Homer-3 expression in Sca1154Q/2Q PCs attenuated cellular dysfunctions and improved motor deficits. Our work reveals that impaired mTORC1-Homer-3 activity underlies PC susceptibility in SCA1 and presents a promising therapeutic target.
“Impaired mTORC1-dependent expression of Homer-3 influences SCA1 pathophysiology” by Céline Ruegsegger, David M. Stucki, Silvio Steiner, Nico Angliker, Julika Radecke, Eva Keller, Benoît Zuber, Markus A. Rüegg and Smita Saxena in SNeuron. Published online January 6 2016 doi:10.1016/j.neuron.2015.11.033






