Ketamine, an anesthetic sometimes abused as a street drug, increases the synaptic connections between brain cells and in low doses acts as a powerful antidepressant, Yale researchers have found. However, stress has the opposite effect, shrinking the number of synaptic spines, triggering depression.
In the April 13 online issue of the journal Nature Medicine, Yale researchers found that expression of single gene called REDD1 enables stress to damage brain cells and cause depressive behavior.
“We found if we delete REDD1, we can block the effects of stress in mice,” said Ron Duman, the Elizabeth Mears and House Jameson Professor of Psychiatry and professor of neurobiology.
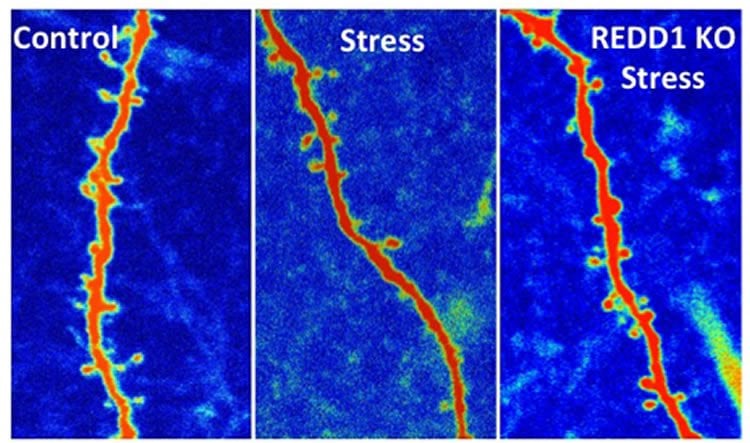
In recent studies, the Yale team showed that ketamine activates the mTORC1 pathway, which in turn spurs synthesis of synaptic proteins and connections. In the new study, they show that the REDD1 gene expression blocks mTORC1 activity and decreases the number of synaptic connections. The new study by Duman and lead author Kristie Ota showed that mice without the REDD1 gene were impervious to the synaptic and behavioral deficits caused by stress. By contrast, when the gene was over-expressed, mice exhibited loss of synaptic connections and increased depression and anxiety behaviors.
In addition, post-mortem examinations of people who had suffered from depression showed high levels of REDD1 in cortical regions associated with depression.
Yale’s work with ketamine has already led to development of new classes of antidepressants, which are currently in clinical trials. Duman said these new findings may provide a new drug target that directly blunts the negative impacts of stress.
Other Yale authors include Rong-Jian Liu, Bhavya Voleti, Jaime G. Maldonado-Aviles, Vanja Duric, Masaaki Iwata, Sophie Dutheil, Catharine Duman. Ralph J. DiLeone, and and George K. Aghajanian.
Funding for research was provided by the National Institutes of Health.
Contact: Bill Hathaway – Yale
Source: Yale press release
Image Source: The image is credited to Duman lab and is adapted from the Yale press release
Original Research: Abstract for “REDD1 is essential for stress-induced synaptic loss and depressive behavior” by Kristie T Ota, Rong-Jian Liu, Bhavya Voleti, Jaime G Maldonado-Aviles, Vanja Duric, Masaaki Iwata, Sophie Dutheil, Catharine Duman, Steve Boikess, David A Lewis, Craig A Stockmeier, Ralph J DiLeone, Christopher Rex, George K Aghajanian and Ronald S Duman in Nature Medicine. Published online April 13 2014 doi:10.1038/nm.3513







