Summary: A new study questions previous assumption about how Parkinson’s disease develops and progresses.
Source: University of Basel.
Parkinson’s disease was first described by a British doctor more than 200 years ago. The exact causes of this neurodegenerative disease are still unknown. In a study recently published in eLife, a team of researchers led by Prof. Henning Stahlberg from the Biozentrum of the University of Basel has now questioned the previous understanding of this disease.
The arms and legs tremble incessantly, the muscles become weaker and the movements slower − these are typical symptoms that many Parkinson’s patients suffer from. More than six million people are affected worldwide. In these patients, the dopamine-producing nerve cells in the brain slowly die off. The resulting lack of this neurotransmitter impairs motor function and often also affects the cognitive abilities.
Questionable: protein fibrils cause Parkinson’s disease
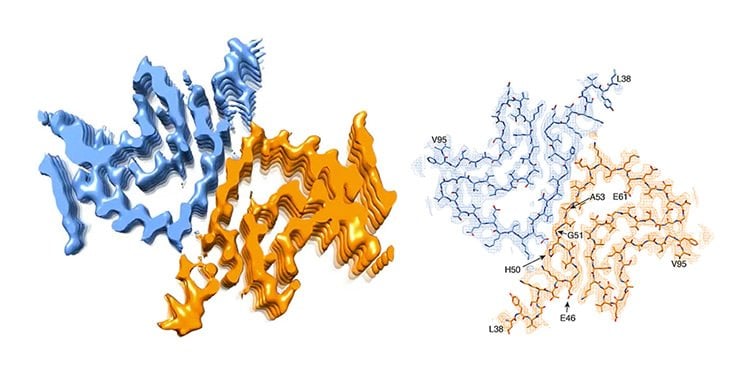
So far, it was assumed that the protein alpha-synuclein is one of the trigger factors. This protein can clump together and form small needles, so-called fibrils, which accumulate and deposit as Lewy bodies in the nerve cells. These toxic fibrils damage the affected brain cells. A team of scientists led by Prof. Henning Stahlberg from the Biozentrum of the University of Basel, in collaboration with researchers from Hoffmann-La Roche Ltd. and the ETH Zurich, have now artificially generated an alpha-synuclein fibril in the test tube. They have been able to visualize for the first time its three-dimensional structure with atomic resolution. “Contrary to our expectations, the results seem to raise more questions than they can hope to answer,” says Stahlberg.
It is important to know that in some congenital forms of Parkinson’s disease, affected persons carry genetic defects in the alpha-synuclein gene. These mutations, it is suspected, eventually cause the protein to fold incorrectly, thus forming dangerous fibrils. “However, our 3D structure reveals that a mutated alpha-synuclein protein should not be able to form these type of fibrils,” says Stahlberg. “Because of their location, most of these mutations would rather hinder the formation of the fibril structure that we have found.” In brief, if the fibril structure causes Parkinson’s disease, the genetic defect would have to protect against the disease. But this is not the case. So, it could be possible that a different type of fibril or another form of the protein triggers the disease in these patients.
Study poses new questions
More investigations are now needed to understand this fibril structure. What are the effects of the alpha-synuclein mutations? Do they lead to distinct forms of protein aggregates? What is the role of the fibrils for the nerve cells, and why do these cells die? To date, the exact physiological function of alpha-synuclein is still not known. Since only the symptoms of this neurodegenerative disease can be alleviated with the current medications, new concepts are urgently needed.
Source: University of Basel
Publisher: Organized by NeuroscienceNews.com.
Image Source: NeuroscienceNews.com image is credited to the researchers.
Original Research: Abstract for “Cryo-EM structure of alpha-synuclein fibrils” by Ricardo Guerrero-Ferreira, Nicholas M I Taylor, Daniel Mona, Philippe Ringler, Matthias E Lauer, Roland Riek, Markus Britschgi, and Henning Stahlberg in eLife. Published July 3 2018.
doi:10.7554/eLife.36402
[cbtabs][cbtab title=”MLA”]University of Basel”Study Raises Doubts on a Previous Theory of Parkinson’s.” NeuroscienceNews. NeuroscienceNews, 6 July 2018.
<https://neurosciencenews.com/parkinsons-theory-9523/>.[/cbtab][cbtab title=”APA”]University of Basel(2018, July 6). Study Raises Doubts on a Previous Theory of Parkinson’s. NeuroscienceNews. Retrieved July 6, 2018 from https://neurosciencenews.com/parkinsons-theory-9523/[/cbtab][cbtab title=”Chicago”]University of Basel”Study Raises Doubts on a Previous Theory of Parkinson’s.” https://neurosciencenews.com/parkinsons-theory-9523/ (accessed July 6, 2018).[/cbtab][/cbtabs]
Abstract
Cryo-EM structure of alpha-synuclein fibrils
Parkinson’s disease is a progressive neuropathological disorder that belongs to the class of synucleopathies, in which the protein alpha-synuclein is found at abnormally high concentrations in affected neurons. Its hallmark are intracellular inclusions called Lewy bodies and Lewy neurites. We here report the structure of cytotoxic alpha-synuclein fibrils (residues 1-121), determined by cryo-electron microscopy structure at a resolution of 3.4Å. Two protofilaments form a polar fibril composed of staggered β-strands. The backbone of residues 38 to 95, including the fibril core and the non-amyloid component region, are well resolved in the EM map. Residues 50-57, containing three of the mutation sites associated with familial synucleinopathies, form the interface between the two protofilaments and contribute to fibril stability. A hydrophobic cleft at one end of the fibril may have implications for fibril elongation, and invites for the design of molecules for diagnosis and treatment of synucleinopathies.