Summary: A new model of Parkinson’s disease demonstrates how abnormal alpha-synuclein proteins gradually spread from brain areas implicated in early stages of the disease to other regions of the brain.
Source: Van Andel Research Institute.
They’re two of the biggest mysteries in Parkinson’s disease research–where does the disease start? And how can it be stopped early in the process?
Now, a new laboratory model of Parkinson’s is giving scientists an inside look at what happens in the brain years before motor symptoms appear. Specifically, it demonstrates how abnormal alpha-synuclein proteins, which are strongly associated with Parkinson’s, gradually spread from an area of the brain implicated in the early stages of the disease to other regions of the brain ultimately damaged by the disease. The findings were published today in the Journal of Experimental Medicine.
Parkinson’s is primarily a disease of aging, with most cases diagnosed after age 60. By the time symptoms appear, more than half of the brain cells that produce dopamine, a chemical messenger needed for voluntary movement, have died. What triggers this process is unknown, although evidence points to a combination of genetic, epigenetic and environmental factors. Strong evidence also suggests that clumps of abnormal alpha-synuclein play a role in the disease process. In recent years, scientists have found links to the early stages of Parkinson’s in other areas of the body, namely the gut and the nose.
“Better models that mimic the early stages of the disease will allow us to more precisely study Parkinson’s and, by extension, find new ways to potentially stop it before it progresses,” said Van Andel Research Institute researcher Nolwen L. Rey, Ph.D., the study’s first author. “We know that specific signs of Parkinson’s, including a loss of sense of smell, appear years before the onset of motor symptoms. Our new model replicates the phase that occurs long before diagnosis and, importantly, gives us a powerful tool to test novel interventions that might prevent the onset of Parkinson’s as we know it.”
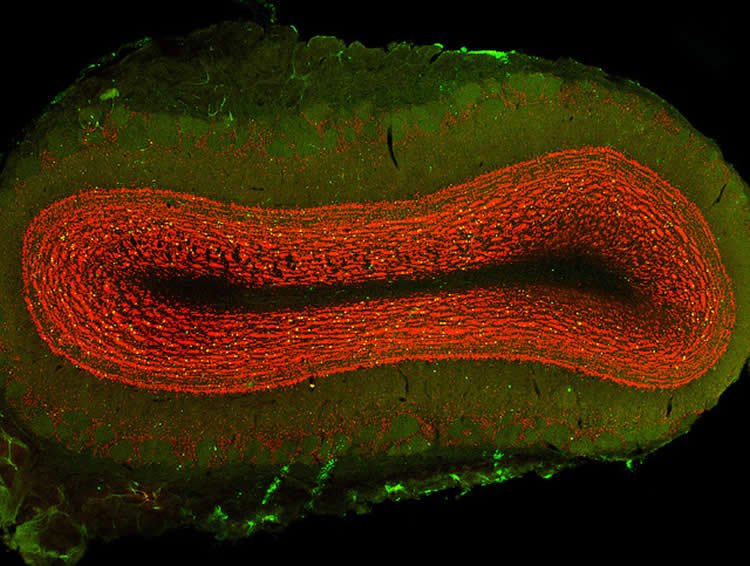
The study demonstrates that alpha-synuclein travels along nerve cells in the olfactory bulb–the part of the brain that controls sense of smell–prior to the onset of motor symptoms and that this area may be particularly susceptible to the spread of alpha-synuclein, ultimately causing deficits in the sense of smell. Clumps of alpha-synuclein eventually reach several additional brain regions, including the brainstem area that houses dopamine cells.
“Perhaps most remarkably, we have created a model of prodromal Parkinson’s disease, the condition that precedes the diagnosis of the disorder in humans by five to 10 years, that successfully mimics the pattern of alpha-synuclein’s pathology in the brain,” said Patrik Brundin, M.D., Ph.D., senior author of the study and director of Van Andel Research Institute’s Center for Neurodegenerative Science. “Not only might this teach us something about how the disease develops and the importance of the olfactory system, but the model will also prove invaluable when testing novel therapeutics designed to slow down or stop the progression of disease.”
Funding: This work was supported by Van Andel Research Institute, the Peter C. and Emajean Cook Foundation, the European Research Council and University of Pennsylvania Morris K. Udall Parkinson’s Disease Research Center of Excellence. Other study authors include Jennifer A. Steiner (Van Andel Research Institute), Nazia Maroof (Roche Innovation Center), Kelvin C. Luk (University of Pennsylvania), Zachary Madaj (Van Andel Research Institute), John Q. Trojanowski (University of Pennsylvania), and Virginia M-Y Lee (University of Pennsylvania).
Source: Beth Hinshaw Hall – Van Andel Research Institute
Image Source: This NeuroscienceNews.com image is credited to Jason Snyder, licensed CC BY 2.0.
Original Research: Abstract for “Widespread transneuronal propagation of α-synucleinopathy triggered in olfactory bulb mimics prodromal Parkinson’s disease” by Rey NL, Steiner JA, Maroof N, Luk KC, Madaj Z, Trojanowski JQ, Lee VMY, and Brundin P. in JEM. Published online August 8 2016 doi:10.1084/jem.20160368
[cbtabs][cbtab title=”MLA”]Van Andel Research Institute. “New Model Recreates Early Spread of Parkinson’s in the Brain.” NeuroscienceNews. NeuroscienceNews, 9 August 2016.
<https://neurosciencenews.com/parkinsons-spread-model-neurology-4809/>.[/cbtab][cbtab title=”APA”]Van Andel Research Institute. (2016, August 9). New Model Recreates Early Spread of Parkinson’s in the Brain. NeuroscienceNews. Retrieved August 9, 2016 from https://neurosciencenews.com/parkinsons-spread-model-neurology-4809/[/cbtab][cbtab title=”Chicago”]Van Andel Research Institute. “New Model Recreates Early Spread of Parkinson’s in the Brain.” https://neurosciencenews.com/parkinsons-spread-model-neurology-4809/ (accessed August 9, 2016).[/cbtab][/cbtabs]
Abstract
Widespread transneuronal propagation of α-synucleinopathy triggered in olfactory bulb mimics prodromal Parkinson’s disease
Parkinson’s disease (PD) is characterized by the progressive appearance of intraneuronal Lewy aggregates, which are primarily composed of misfolded α-synuclein (α-syn). The aggregates are believed to propagate via neural pathways following a stereotypical pattern, starting in the olfactory bulb (OB) and gut. We hypothesized that injection of fibrillar α-syn into the OB of wild-type mice would recreate the sequential progression of Lewy-like pathology, while triggering olfactory deficits. We demonstrate that injected α-syn fibrils recruit endogenous α-syn into pathological aggregates that spread transneuronally over several months, initially in the olfactory network and later in distant brain regions. The seeded inclusions contain posttranslationally modified α-syn that is Thioflavin S positive, indicative of amyloid fibrils. The spreading α-syn pathology induces progressive and specific olfactory deficits. Thus, we demonstrate that propagating α-syn pathology triggered in the OB is functionally detrimental. Collectively, we have created a mouse model of prodromal PD.
“Widespread transneuronal propagation of α-synucleinopathy triggered in olfactory bulb mimics prodromal Parkinson’s disease” by Rey NL, Steiner JA, Maroof N, Luk KC, Madaj Z, Trojanowski JQ, Lee VMY, and Brundin P. in JEM. Published online August 8 2016 doi:10.1084/jem.20160368