Summary: Stimulating mouse neurons in a dish lead to a build-up to fatty acids and lipid particle release. Astrocytes engulfed the particles and increased genetic activity associated with detoxification.
Source: HHMI
Astrocytes are overtaxed neurons’ pit crew.
The brain cells collect damaged lipids secreted by hyperactive neurons, then recycle those toxic molecules into energy, researchers at the Howard Hughes Medical Institute’s Janelia Research Campus report May 23, 2019, in the journal Cell. It’s a mechanism to protect neurons from the damaging side effects of overactivity. And it’s another important role for astrocytes, which support neurons in various ways.
When a neuron fires fast and furious, lipid molecules in the cell get damaged and can become toxic. While most kinds of cells sequester excess fatty acids away or feed them to mitochondria to prevent buildup, neurons don’t seem to rely on those tricks.
Instead, “neurons unload some of the burden to astrocytes,” says study coauthor and Janelia Group Leader Zhe Liu, who worked closely with Maria Ioannou and Jennifer Lippincott-Schwartz, a senior group leader at Janelia. “For a long time, people have suspected there was some mechanism like this. The new work shows how this process actually happens.”
The finding arose from a curious observation: Overactive neurons release damaged fatty acids bundled up in lipid particles.
“People didn’t think that neurons could secrete those lipid particles,” Liu says.
But stimulating mouse neurons in a dish led to the buildup of fatty acids and, eventually, lipid particle release, the team showed. Then, nearby astrocytes engulfed the particles and amped up the activity of genes involved in energy production and detoxification.
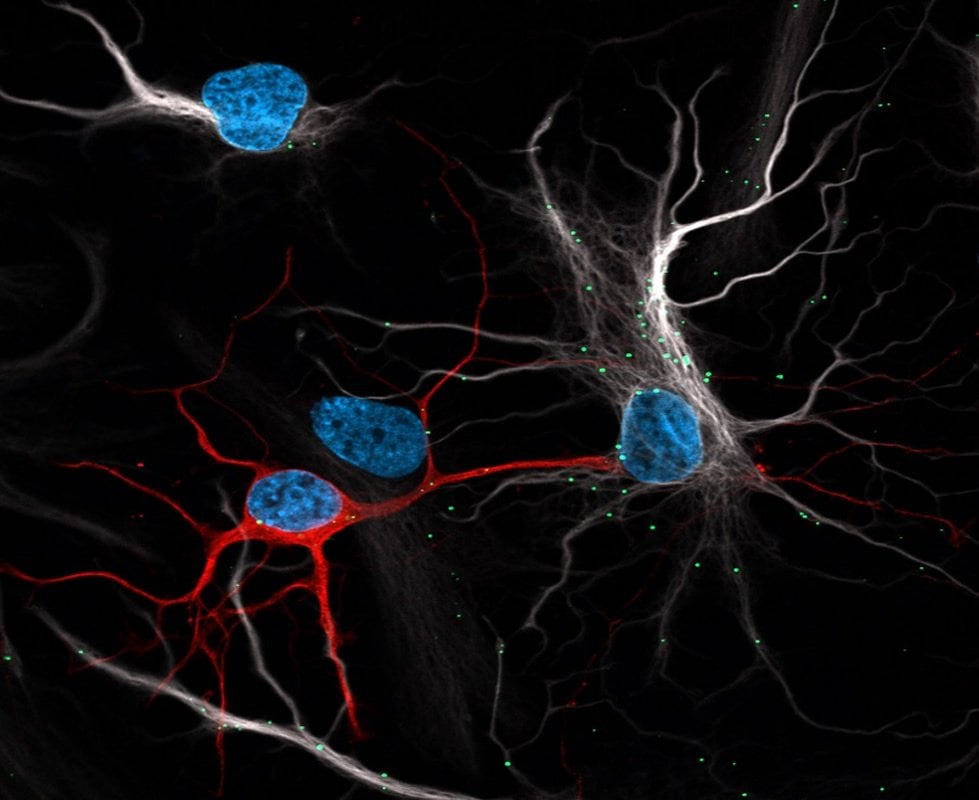
Astrocytes feed neurons’ off-loaded damaged lipids to their own mitochondria, converting waste into energy, Liu concluded. Tests in mice showed a similar response. After a lesion to the brain that mimics a stroke – a huge stress to neurons – neurons increased production of proteins involved in transporting fatty acids out of the cell, and fatty acids built up in astrocytes.
This pathway for clearing toxic molecules from neurons might be damaged in Alzheimer’s patients, Liu proposes, though that hasn’t been thoroughly investigated. A next step, led by Maria Ioannou in her new lab at the University of Alberta, is to examine what’s different about this mechanism in cell culture and rodent models of Alzheimer’s disease.
Source:
HHMI
Media Contacts:
Laurel Hamers – HHMI
Image Source:
The image is credited to Maria Ioannou.
Original Research: Closed access
“Neuron-Astrocyte Metabolic Coupling Protects against Activity-Induced Fatty Acid Toxicity”. Maria Ioannou et al.
Cell. doi:10.1016/j.cell.2019.04.001
Abstract
Neuron-Astrocyte Metabolic Coupling Protects against Activity-Induced Fatty Acid Toxicity
Highlights
• Hyperactive neurons release excess FAs in lipid particles associated with ApoE
• Astrocytes endocytose neuron-derived lipid particles, delivering the FAs to LDs
• Astrocytes with LDs upregulate metabolic and detoxification genes
• Neural activity triggers astrocytic consumption of FAs by mitochondrial oxidation
Summary
Metabolic coordination between neurons and astrocytes is critical for the health of the brain. However, neuron-astrocyte coupling of lipid metabolism, particularly in response to neural activity, remains largely uncharacterized. Here, we demonstrate that toxic fatty acids (FAs) produced in hyperactive neurons are transferred to astrocytic lipid droplets by ApoE-positive lipid particles. Astrocytes consume the FAs stored in lipid droplets via mitochondrial β-oxidation in response to neuronal activity and turn on a detoxification gene expression program. Our findings reveal that FA metabolism is coupled in neurons and astrocytes to protect neurons from FA toxicity during periods of enhanced activity. This coordinated mechanism for metabolizing FAs could underlie both homeostasis and a variety of disease states of the brain.






