Summary: A newly developed hydrogel scaffold with regularly spaced pores assists in spinal cell growth and neuron regeneration following spinal cord injury.
Source: American Institute of Physics
Spinal cord injuries can be life-changing and alter many important neurological functions. Unfortunately, clinicians have relatively few tools to help patients regain lost functions.
In APL Bioengineering, by AIP Publishing, researchers from UCLA have developed materials that can interface with an injured spinal cord and provide a scaffolding to facilitate healing. To do this, scaffolding materials need to mimic the natural spinal cord tissue, so they can be readily populated by native cells in the spinal cord, essentially filling in gaps left by injury.
“In this study, we demonstrate that incorporating a regular network of pores throughout these materials, where pores are sized similarly to normal cells, increases infiltration of cells from spinal cord tissue into the material implant and improves regeneration of nerves throughout the injured area,” said author Stephanie Seidlits.
The researchers show how the pores improve efficiency of gene therapies administered locally to the injured tissues, which can further promote tissue regeneration.
Since many spinal cord injuries result from a contusion, the biomaterial implants need to be injected in or near the injured area without causing damage to any surrounding spared tissue. A major technical challenge has been fabricating scaffold materials with cell-scale pore sizes that are also injectable.
In the researchers’ method, they injected beads of material through a small needle into the spinal cord, where the beads stick together to form a scaffold, where cells can crawl in the pore spaces between the beads. The researchers found inclusion of these larger cell-scale pores within biomaterial scaffolds improved cell infiltration, gene delivery, and tissue repair after spinal cord injury, compared to more conventional materials with nanoscale pores.
The researchers made the highly porous scaffolds using two different methods. One was simpler but created a more irregularly sized pore network. The second was more complicated but created a highly regular pore structure.
Even though both materials had the same average pore size and chemical composition, more regenerating nerves were found to infiltrate scaffolds with regularly shaped pores.
“These results inform how to maximize interfacing with the nervous system,” said Seidlits. “This has potential applications not only for developing new therapies for brain and spinal cord repair but also for brain-machine interfaces, prosthetics, and treatment of neurodegenerative diseases.”
About this SCI research news
Source: American Institute of Physics
Contact: Larry Frum – American Institute of Physics
Image: The image is credited to Seidlits et al.
Original Research: Open access.
“Injectable, macroporous scaffolds for delivery of therapeutic genes to the injured spinal cord” by Seidlits et al. APL Bioengineering
Abstract
Injectable, macroporous scaffolds for delivery of therapeutic genes to the injured spinal cord
Biomaterials are being developed as therapeutics for spinal cord injury (SCI) that can stabilize and bridge acute lesions and mediate the delivery of transgenes, providing a localized and sustained reservoir of regenerative factors.
For clinical use, direct injection of biomaterial scaffolds is preferred to enable conformation to unique lesions and minimize tissue damage. While an interconnected network of cell-sized macropores is necessary for rapid host cell infiltration into—and thus integration of host tissue with—implanted scaffolds, injectable biomaterials have generally suffered from a lack of control over the macrostructure.
As genetic vectors have short lifetimes in vivo, rapid host cell infiltration into scaffolds is a prerequisite for efficient biomaterial-mediated delivery of transgenes. We present scaffolds that can be injected and assembled in situ from hyaluronic acid (HA)-based, spherical microparticles to form scaffolds with a network of macropores (∼10 μm).
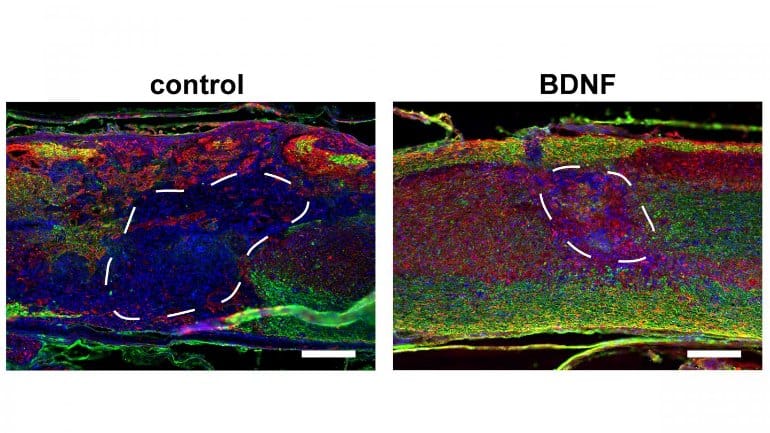
The results demonstrate that addition of regularly sized macropores to traditional hydrogel scaffolds, which have nanopores (∼10 nm), significantly increases the expression of locally delivered transgene to the spinal cord after a thoracic injury. Maximal cell and axon infiltration into scaffolds was observed in scaffolds with more regularly sized macropores.
The delivery of lentiviral vectors encoding the brain-derived neurotrophic factor (BDNF), but not neurotrophin-3, from these scaffolds further increased total numbers and myelination of infiltrating axons. Modest improvements to the hindlimb function were observed with BDNF delivery.
The results demonstrate the utility of macroporous and injectable HA scaffolds as a platform for localized gene therapies after SCI.