Summary: A new tool allows researchers to observe granulins inside cells.
Source: Emory Health Sciences.
Emory University School of Medicine researchers have developed tools that enable them to detect small proteins called granulins for the first time inside cells. Granulins are of interest to neuroscientists because mutations in the granulin gene cause frontotemporal dementia (FTD). However, the functions of granulins were previously unclear.
FTD is an incurable neurodegenerative disease and the most common type of dementia in people younger than 60. Genetic variants in the granulin gene are also a risk factor for Alzheimer’s disease and Parkinson’s disease, suggesting this discovery may have therapeutic potential for a broad spectrum of age-related neurodegenerative diseases.
The results were published August 9 by the journal eNeuro.
Some neuroscientists believed that granulins were made outside cells, and even could be toxic under certain conditions. But with the newly identified tools, the Emory researchers can now see granulins inside cells within lysosomes, which are critical garbage disposal and recycling centers. The researchers now propose that granulins have important jobs in the lysosome that are necessary to maintain brain health, suppress neuroinflammation, and prevent neurodegeneration.
Problems with lysosomes appear in several neurodegenerative diseases such as Alzheimer’s and Parkinson’s.
“A lysosomal function for granulins is exciting and novel. We believe it may provide an explanation why decreased levels of granulins are linked to multiple neurodegenerative diseases, ranging from frontotemporal dementia to Alzheimer’s,” says senior author Thomas Kukar, PhD, assistant professor of pharmacology and neurology and the Emory University Center for Neurodegenerative Disease.
Granulins are derived from a larger precursor protein called progranulin. A genetic deficiency in progranulin explains some forms of frontotemporal dementia (FTD), both familial and sporadic.
If both copies of the progranulin gene are mutated, the result is a rare disorder: neuronal ceroid lipofuscinosis (NCL). NCL is a member of the larger group of lysosomal storage diseases (LSDs), inherited diseases caused by defects in lysosome function. NCL can be caused by mutations in several genes encoding lysosomal proteins. NCL is partly named after lipofuscins – deposits of fats and proteins – which accumulate inside cells because of impaired lysosomes. NCL typically appears earlier in life, with features such as blindness, developmental delay, motor problems and seizures.
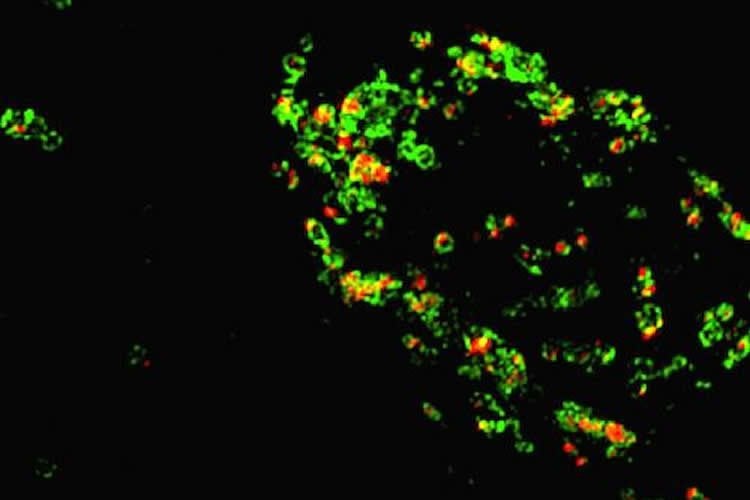
“Over the past few years, evidence has accumulated that progranulin deficiency impairs the function of lysosomes, but scientists still don’t know why”, says assistant scientist Chris Holler, PhD, the first author of the paper.
“Although some people suspected that progranulin gets cleaved in the lysosome, it could not be proven,” he says. “Our paper is the first to actually show that mature granulins are made in the lysosome.”
“More importantly, the granulins are stable in lysosomes suggesting they have an important function, rather than just an inert by-product”, he adds. The next step is to use the antibody tools to probe what granulins are doing in lysosomes. Granulins may work together with similar lysosomal caretakers called saposins, Holler says.
The findings point to a strategy for treating diseases caused by progranulin deficiency (both FTD and NCL, possibly) by supplying replacement granulins, either as proteins or through gene therapy. The protein/enzyme replacement approach is already used for other forms of NCL and LSDs.
Co-authors include research specialist Georgia Taylor and post-doc Qiudong Deng, PhD.
A patent related to this work titled “Methods to Treat Neurodegeneration with Granulins” has been filed. As inventors, the authors could benefit from future commercialization.
Funding: The research was supported by the National Institute on Aging (R00AG032362, P50AG025688), the National Institute of Neurological Disorders and Stroke (R01NS093362), the Donors Cure Foundation, the Alzheimer’s Association, the Association for Frontotemporal Degeneration, and the Bluefield Project to Cure Frontotemporal Dementia.
Source: Quinn Eastman – Emory Health Sciences
Image Source: NeuroscienceNews.com image is credited to Chris Holler, Emory University School of Medicine.
Original Research: Abstract for “Intracellular Proteolysis of Progranulin Generates Stable, Lysosomal Granulins That Are Haploinsufficient in Patients with Frontotemporal Dementia Caused by GRN Mutations” by Christopher J. Holler, Georgia Taylor, Qiudong Deng and Thomas Kukar in eNeuro. Published online MAugust 9 2017 doi:10.1523/ENEURO.0100-17.2017
[cbtabs][cbtab title=”MLA”]Emory Health Sciences “Granulins Are Brain Treasure, Not Trash.” NeuroscienceNews. NeuroscienceNews, 15 August 2017.
<https://neurosciencenews.com/granulins-neuroscience-7313/>.[/cbtab][cbtab title=”APA”]Emory Health Sciences (2017, August 15). Granulins Are Brain Treasure, Not Trash. NeuroscienceNew. Retrieved August 15, 2017 from https://neurosciencenews.com/granulins-neuroscience-7313/[/cbtab][cbtab title=”Chicago”]Emory Health Sciences “Granulins Are Brain Treasure, Not Trash.” https://neurosciencenews.com/granulins-neuroscience-7313/ (accessed August 15, 2017).[/cbtab][/cbtabs]
Abstract
Intracellular Proteolysis of Progranulin Generates Stable, Lysosomal Granulins That Are Haploinsufficient in Patients with Frontotemporal Dementia Caused by GRN Mutations
Homozygous or heterozygous mutations in the GRN gene, encoding progranulin (PGRN), cause neuronal ceroid lipofuscinosis (NCL) or frontotemporal dementia (FTD), respectively. NCL and FTD are characterized by lysosome dysfunction and neurodegeneration, indicating PGRN is important for lysosome homeostasis in the brain. PGRN is trafficked to the lysosome where its functional role is unknown. PGRN can be cleaved into seven 6 kDa proteins called granulins (GRNs), however little is known about how GRNs are produced or if levels of GRNs are altered in FTD-GRN mutation carriers. Here, we report the identification and characterization of antibodies that reliably detect several human GRNs by immunoblot and immunocytochemistry. Using these tools, we find that endogenous GRNs are present within multiple cell lines and are constitutively produced. Further, extracellular PGRN is endocytosed and rapidly processed into stable GRNs within lysosomes. Processing of PGRN into GRNs is conserved between humans and mice and is modulated by sortilin expression and cysteine protease activity. Induced lysosome dysfunction caused by alkalizing agents or increased expression of TMEM106B inhibit processing of PGRN into GRNs. Finally, we find that multiple GRNs are haploinsufficient in primary fibroblasts and cortical brain tissue from FTD-GRN patients. Taken together, our findings raise the interesting possibility that GRNs carry out critical lysosomal functions and that loss of GRNs should be explored as an initiating factor in lysosomal dysfunction and neurodegeneration caused by GRN mutations.
Significance Statement Progranulin (PGRN) plays a critical, yet undefined role in lysosome function. PGRN is cleaved into 6kDa proteins called granulins (GRNs), but this process is poorly understood. We find that PGRN is proteolytically processed into stable, lysosomal GRNs, implying that GRNs may have a functional role in the lysosome, and are not toxic as previously proposed. Moreover, deficiency of GRNs in frontotemporal dementia (FTD) caused by GRN mutations may play a causal role in the development of lysosome dysfunction that underlies FTD-GRN, which paves the way for testing GRN replacement as a therapeutic strategy. Finally, potential drug candidates to treat FTD-GRN should evaluate their effect on the production of both PGRN and GRNs in the brain.
“Intracellular Proteolysis of Progranulin Generates Stable, Lysosomal Granulins That Are Haploinsufficient in Patients with Frontotemporal Dementia Caused by GRN Mutations” by Christopher J. Holler, Georgia Taylor, Qiudong Deng and Thomas Kukar in eNeuro. Published online MAugust 9 2017 doi:10.1523/ENEURO.0100-17.2017