Summary: A new study reports herpes virus utilizes ancient RNA to proliferate, mimicking the same process tumors have been found to manipulate. The findings could have implications for new treatment options and also may shed light on neurodegenerative diseases.
Source: Mount Sinai Hospital.
Herpes viral infections use the ancient genetic material found in the human genome to proliferate, mimicking the same process tumors have been found to manipulate, Mount Sinai researchers have shown for the first time. These observations provide further insight about how herpes viruses can manipulate the immune system in ways that may drive neurodegenerative diseases like Alzheimer’s, according to the study, published in Nature Communications in January.
The researchers found that herpes viruses appear to manipulate an ancient RNA species that originated several million years ago, called human satellite II RNA (HSATII RNA). HSATII RNA is normally inactive, but both herpes viruses and cancer cells have essentially learned to activate it, using this RNA to manipulate their environment to help them invade the body and grow.
The researchers believe that both viruses and cancer cells figured out how to use this RNA because they both rapidly evolve to test out different strategies to multiply and spread within the body over time. Researchers have yet to understand whether herpes and cancer came upon this strategy coincidentally or whether they work hand in hand in some cases. Several researchers involved in this work pioneered the study of how a different type of RNA affects tumor evolution.
“The evolution of tumors can teach us about viruses and vice versa, and understanding one system may help us treat the other,” said one of the study’s senior authors, Benjamin Greenbaum, PhD, Assistant Professor of Oncological Sciences, Pathology, and Medicine (Hematology and Medical Oncology) at The Tisch Cancer Institute at the Icahn School of Medicine at Mount Sinai. “The HSATII RNA induction seen in herpes infections and cancer cells suggests possible convergence upon common mechanisms in these seemingly disparate diseases.”
The study potentially gives further insight into how herpes viruses might play a role in developing colitis and neurodegenerative diseases like Alzheimer’s. It is the first step toward potentially developing diagnostic tools that look for these types of RNAs in cancer and herpes patients and using the ancient RNAs as targets for drugs in the future, said Dr. Greenbaum.
The lead author of the study was Maciej Nogalski, PhD, Postdoctoral Research Fellow in the laboratory of co-senior author Thomas Shenk, PhD, James A. Elkins Professor of Life Sciences in the Department of Molecular Biology at Princeton University.
“Herpes viruses have been extensively studied for many years, but once again by investigating host-virus interactions at the cellular level we were privileged to get insights into novel regulatory mechanisms of human cells. Our virus-centered studies not only uncovered interesting aspects of viral infection, but also provided an inducible system that could accelerate investigations about possible roles HSATII RNA plays in other diseases,” Dr. Nogalski says.
Funding: Researchers from Massachusetts General Hospital and the Simons Center for Systems Biology at the Institute for Advanced Study also contributed to this work. This research received funding from the National Institutes of Health (AI112951), the American Cancer Society(PF-14-116-01 MPC), the V Foundation, Stand Up to Cancer, the National Science Foundation, and the Lustgarten Foundation, the Pershing Square Sohn Research Alliance, the Mark Foundation, the Burroughs Wellcome Fund, and Affymetrix, Inc.
Source: Marlene Naanes – Mount Sinai Hospital
Publisher: Organized by NeuroscienceNews.com.
Image Source: NeuroscienceNews.com image is credited to Greenbaum et al./Nature Communications.
Original Research: Open access research for “A tumor-specific endogenous repetitive element is induced by herpesviruses” by Maciej T. Nogalski, Alexander Solovyov, Anupriya S. Kulkarni, Niyati Desai, Adam Oberstein, Arnold J. Levine, David T. Ting, Thomas Shenk & Benjamin D. Greenbaum in Nature Communications. Published January 9 2019.
doi:10.1038/s41467-018-07944-x
[cbtabs][cbtab title=”MLA”]Mount Sinai Hospital”Herpes Virus and Tumors Evolved to Learn How to Manipulate the Same Ancient RNA.” NeuroscienceNews. NeuroscienceNews, 14 January 2019.
<https://neurosciencenews.com/cancer-herpes-rna-10539/>.[/cbtab][cbtab title=”APA”]Mount Sinai Hospital(2019, January 14). Herpes Virus and Tumors Evolved to Learn How to Manipulate the Same Ancient RNA. NeuroscienceNews. Retrieved January 14, 2019 from https://neurosciencenews.com/cancer-herpes-rna-10539/[/cbtab][cbtab title=”Chicago”]Mount Sinai Hospital”Herpes Virus and Tumors Evolved to Learn How to Manipulate the Same Ancient RNA.” https://neurosciencenews.com/cancer-herpes-rna-10539/ (accessed January 14, 2019).[/cbtab][/cbtabs]
Abstract
A tumor-specific endogenous repetitive element is induced by herpesvirusese
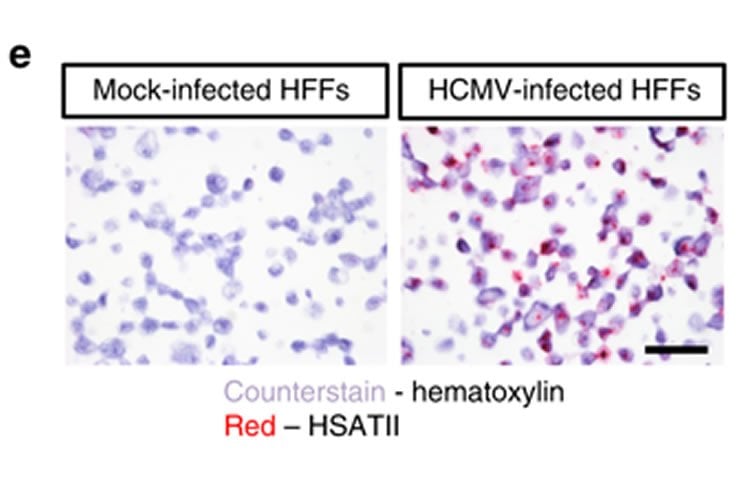
Tandem satellite repeats account for 3% of the human genome. One of them, Human Satellite II (HSATII), is highly expressed in several epithelial cancers and cancer cell lines. Here we report an acute induction of HSATII RNA in human cells infected with two herpes viruses. We show that human cytomegalovirus (HCMV) IE1 and IE2 proteins cooperate to induce HSATII RNA affecting several aspects of the HCMV replication cycle, viral titers and infected-cell processes. HSATII RNA expression in tissue from two chronic HCMV colitis patients correlates with the strength of CMV antigen staining. Thus, endogenous HSATII RNA synthesis after herpesvirus infections appears to have functionally important consequences for viral replication and may provide a novel insight into viral pathogenesis. The HSATII induction seen in both infected and cancer cells suggests possible convergence upon common HSATII-based regulatory mechanisms in these seemingly disparate diseases.