Summary: MEF2C, a gene critical for brain development and regulating circuit formation in the brain also plays a significant role in inner ear development. Mutations of MEF2C have previously been linked to ASD. Researchers found mice with only one copy of the MEF2C gene had reduced activity in the auditory nerve.
Source: Medical University of South Carolina
A cross-disciplinary team of researchers in the College of Medicine at the Medical University of South Carolina (MUSC) has discovered hearing impairment in a preclinical model of autism spectrum disorder (ASD).
More specifically, the researchers report in the Journal of Neuroscience that they observed mild hearing loss and defects in auditory nerve function.
Closer examination of the nerve tissue revealed abnormal supportive cells called glia, aging-like degeneration and inflammation. The findings from this study highlight the importance of considering sensory organs and their interactions with the brain in understanding ASD.
Many patients with ASD show increased sensitivity to sound. While many scientists in the past have looked to the brain for an underlying cause, the MUSC team took a different approach by studying the peripheral hearing system.
“Hearing impairment may have an impact on the higher-level auditory system and, eventually, cognitive function,” said Hainan Lang, M.D., Ph.D., professor in the Department of Pathology and Laboratory Medicine at MUSC and one of two senior authors of the study. Jeffrey Rumschlag, Ph.D., a postdoctoral researcher in the MUSC Hearing Research Program, is a co-first author of the manuscript.
Previous studies of aging-related hearing loss showed that the brain can increase its response to make up for reduced auditory signals from the inner ear. Lang wanted to find out if this increase, called central gain, could contribute to abnormal brain response to sound in ASD. However, a significant obstacle lay in her path.
“We didn’t have a clinically relevant model to directly test this important fundamental question,” she said.
The preclinical model that would allow Lang to test her hypothesis was developed in the lab of Christopher Cowan, Ph.D., chair of Neuroscience at MUSC. Mice in this model have only one working copy of a gene called MEF2C. Cowan’s group had studied MEF2C in the past for its role in brain development and found that it was important for regulating circuit formation in the brain.
They became especially interested in creating a preclinical model when a group of patients with ASD-like symptoms were identified with MEF2C mutations. Cowan’s models also show ASD-like behaviors, including increased activity, repetitive behavior and communication deficits.
Lang and Cowan’s collaboration began as they presented posters side by side at an orientation for the College of Graduate Studies at MUSC. Lang’s lab had identified molecular regulators, including MEF2C, crucial for inner ear development, and she saw Cowan’s model as something she could use to test her hypothesis about hearing loss in neurodevelopmental diseases. Cowan enthusiastically agreed, and the research team began to assess the ability of the MEF2C-deficient mice to hear.
They first measured the response of the brain to auditory signals, using a modified version of a test that is commonly used to screen newborn infants for hearing loss. Mild hearing loss was observed in the mice with only one working copy of MEF2C while hearing remained normal in those with two working copies.
To investigate this loss further, the researchers measured the activity of the auditory nerve, which carries signals from the inner ear to the brain. They found reduced activity in this nerve in mice with only one copy of MEF2C.
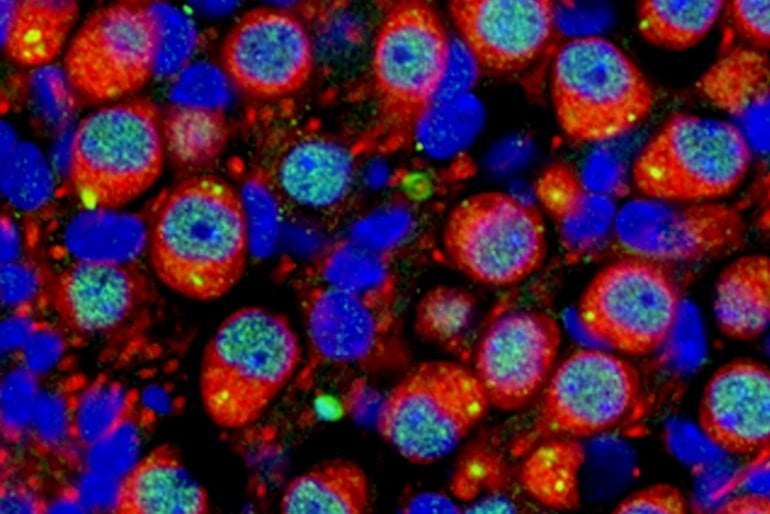
With their sights set on the auditory nerve, the researchers used advanced microscopes and staining techniques to determine what was going wrong. Although the overall hearing sensitivity loss was mild, the researchers were excited to see a big difference in auditory nerve response.
Nerves from mice with a single copy of MEF2C showed cellular degeneration much like that seen in age-related hearing loss. The researchers also saw signs of increased inflammation, with disrupted blood vessels and activated immune cells called glia and macrophages. This finding was especially surprising to the researchers.
“Glial cells were not my first thought; I thought it was a neuronal change,” said Lang. “Now we understand that auditory nerve activity can also involve the immune system, and that’s the beautiful new direction we want to continue to study.”
Cowan also believes that the finding opens the way for a new area of neuroscience research.
“We have more appreciation now that there is an important interaction between the immune system in your body and the immune system in your brain,” he said. “The two systems play critical roles in shaping how nervous system cells communicate with each other, in part, by pruning excess or inappropriate connections that have formed, and this is an essential aspect of healthy brain development and function.”
The findings from this study could be important not only for patients who are MEF2C deficient but also for people with ASD or hearing loss as a whole.
“Understanding how this gene may be participating in ear development and how the inner ear development is affecting brain development has tremendous applicability,” said Cowan.
In future studies, the researchers aim to discover how exactly MEF2C causes the changes that were identified in this study. The research team also hopes to explore these findings in patients with MEF2C deficiency using noninvasive hearing tests.
Lang and Cowan both emphasize the importance of collaboration across disciplines for allowing studies like this to take place.
“The power of collaboration is tremendous for a place like MUSC,” said Cowan. “This collaboration, for us, was ideal because Dr. Lang is an expert in hearing function and development, whereas I am more the genetics and molecular development person. These kinds of collaborations are ideal, and it’s precisely what MUSC is encouraging a lot of us to think about doing more and more.”
“In other words, we each play different instruments so, together, we can make a better harmony,” said Lang.
About this ASD and auditory neuroscience research news
Author: Kimberly McGhee
Source: Medical University of South Carolina
Contact: Kimberly McGhee – Medical University of South Carolina
Image: The image is credited to Dr. Hainan Lang, Medical University of South Carolina
Original Research: Closed access.
“Peripheral auditory nerve impairment in a mouse model of syndromic autism” by Christopher Cowan et al. Journal of Neuroscience
Abstract
Peripheral auditory nerve impairment in a mouse model of syndromic autism
Dysfunction of the peripheral auditory nerve (AN) contributes to dynamic changes throughout the central auditory system, resulting in abnormal auditory processing, including hypersensitivity.
Altered sound sensitivity is frequently observed in autism spectrum disorder (ASD), suggesting that AN deficits and changes in auditory information processing may contribute to ASD-associated symptoms, including social communication deficits and hyperacusis.
The MEF2C transcription factor is associated with risk for several neurodevelopmental disorders, and mutations or deletions of MEF2C produce a haploinsufficiency syndrome characterized by ASD, language, and cognitive deficits.
A mouse model of this syndromic ASD (Mef2c-Het) recapitulates many of the MEF2C haploinsufficiency syndrome-linked behaviors, including communication deficits. We show here that Mef2c-Het mice of both sexes exhibit functional impairment of the peripheral AN and a modest reduction in hearing sensitivity.
We find that MEF2C is expressed during development in multiple AN and cochlear cell types; and in Mef2c-Het mice, we observe multiple cellular and molecular alterations associated with the AN, including abnormal myelination, neuronal degeneration, neuronal mitochondria dysfunction, and increased macrophage activation and cochlear inflammation.
These results reveal the importance of MEF2C function in inner ear development and function and the engagement of immune cells and other non-neuronal cells, which suggests that microglia/macrophages and other non-neuronal cells might contribute, directly or indirectly, to AN dysfunction and ASD-related phenotypes.
Finally, our study establishes a comprehensive approach for characterizing AN function at the physiological, cellular, and molecular levels in mice, which can be applied to animal models with a wide range of human auditory processing impairments.
SIGNIFICANCE STATEMENT
This is the first report of peripheral auditory nerve (AN) impairment in a mouse model of human MEF2C haploinsufficiency syndrome that has well-characterized ASD-related behaviors, including communication deficits, hyperactivity, repetitive behavior, and social deficits.
We identify multiple underlying cellular, subcellular, and molecular abnormalities that may contribute to peripheral AN impairment.
Our findings also highlight the important roles of immune cells (e.g., cochlear macrophages) and other non-neuronal elements (e.g., glial cells and cells in the stria vascularis) in auditory impairment in ASD.
The methodological significance of the study is the establishment of a comprehensive approach for evaluating peripheral AN function and impact of peripheral AN deficits with minimal hearing loss.







