Summary: Injections of IL-33 appear to rescue contextual memories and reduce amyloid beta deposits in mouse models of Alzheimer’s disease.
Source: Hong Kong Institute of Science and Technology.
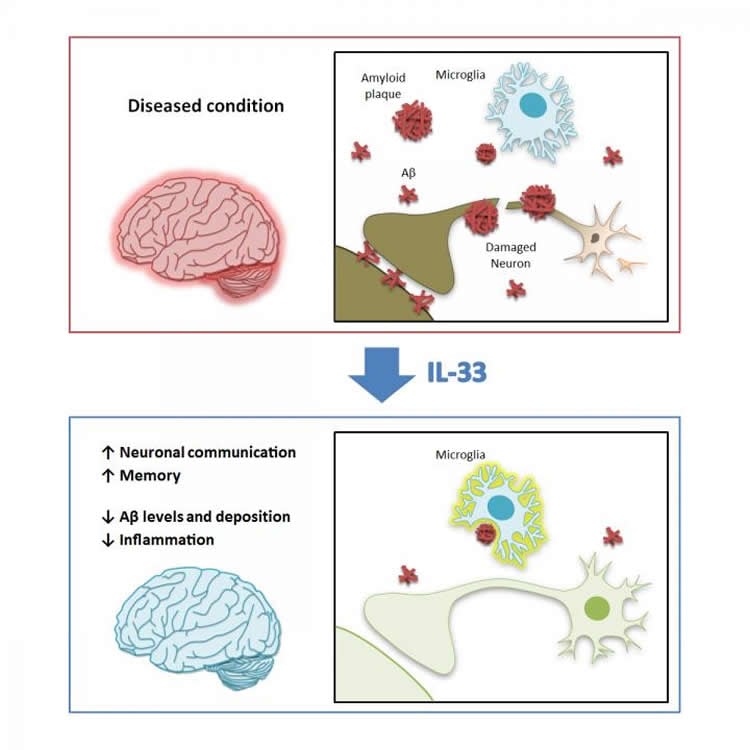
Alzheimer’s disease (AD) is a devastating condition with no known effective treatment. The disease is characterized by memory loss as well as impaired locomotor ability, reasoning, and judgment. Emerging evidence suggests that the innate immune response plays a major role in the pathogenesis of AD.
While the mechanisms underlying the onset and progression of AD remain unclear, scientists from the Hong Kong University of Science and Technology (HKUST) recently conducted a study on the potential therapeutic role of interleukin-33 (IL-33) in AD, where they injected the protein into transgenic mouse models of AD. The injection of IL-33 rescues contextual memory deficits and reduces the deposition of β-amyloid peptide (Aβ) in the transgenic mouse model, suggesting that IL-33 can be developed as a new therapeutic intervention for AD.
The findings were published in the journal PNAS.
“There is no effective therapy for AD, in part because of our limited knowledge of its underlying pathophysiological mechanisms,” said Prof Nancy Ip, Dean of Science, Director of the State Key Laboratory of Molecular Neuroscience and The Morningside Professor of Life Science at HKUST, who directed the research effort. “Nonetheless, targeting the innate immune system has been considered a promising strategy for developing effective therapeutics for AD. The present study demonstrates that peripheral IL-33 injection in AD mouse models alleviates AD-like pathology by enhancing microglial phagocytosis and degradation of Aβ.”
“We believe that IL-33 is a critical factor in maintaining a healthy brain,” Prof Ip said. “Disturbances in this signal mechanism, owing to genetic disposition or environmental influence, may contribute to the onset of AD. The next step will be to translate the findings from the mouse study into clinical treatments for humans.”
The research was the result of a collaborative effort among scientists from HKUST, the University of Glasgow, and Zhejiang University.
Funding: The Research Grants Council of Hong Kong SAR, National Key Basic Research Program of China, Hong Kong Research Grants Council, and S. H. Ho Foundation supported the research.
Source: Sherry No – Hong Kong Institute of Science and Technology
Image Source: This NeuroscienceNews.com image is credited to Division of Life Science, HKUST.
Original Research Full open access research “IL-33 ameliorates Alzheimer’s disease-like pathology and cognitive decline” by Amy K. Y. Fu, Kwok-Wang Hung, Michael Y. F. Yuen, Xiaopu Zhou, Deejay S. Y. Mak, Ivy C. W. Chan, Tom H. Cheung, Baorong Zhang, Wing-Yu Fu, Foo Y. Liew, and Nancy Y. Ip in PNAS. Published online May 10 2016 doi:10.1073/pnas.1604032113
[cbtabs][cbtab title=”MLA”]Hong Kong Institute of Science and Technology. “IL-33 Ameliorates Alzheimer’s-Like Pathology and Cognitive Decline.” NeuroscienceNews. NeuroscienceNews, 6 June 2016.
<https://neurosciencenews.com/alzheimers-il33-neurology-4390/>.[/cbtab][cbtab title=”APA”]Hong Kong Institute of Science and Technology. (2016, June 6). IL-33 Ameliorates Alzheimer’s-Like Pathology and Cognitive Decline. NeuroscienceNews. Retrieved June 6, 2016 from https://neurosciencenews.com/alzheimers-il33-neurology-4390/[/cbtab][cbtab title=”Chicago”]Hong Kong Institute of Science and Technology. “IL-33 Ameliorates Alzheimer’s-Like Pathology and Cognitive Decline.” https://neurosciencenews.com/alzheimers-il33-neurology-4390/ (accessed June 6, 2016).[/cbtab][/cbtabs]
Abstract
IL-33 ameliorates Alzheimer’s disease-like pathology and cognitive decline
Alzheimer’s disease (AD) is a devastating condition with no known effective treatment. AD is characterized by memory loss as well as impaired locomotor ability, reasoning, and judgment. Emerging evidence suggests that the innate immune response plays a major role in the pathogenesis of AD. In AD, the accumulation of β-amyloid (Aβ) in the brain perturbs physiological functions of the brain, including synaptic and neuronal dysfunction, microglial activation, and neuronal loss. Serum levels of soluble ST2 (sST2), a decoy receptor for interleukin (IL)-33, increase in patients with mild cognitive impairment, suggesting that impaired IL-33/ST2 signaling may contribute to the pathogenesis of AD. Therefore, we investigated the potential therapeutic role of IL-33 in AD, using transgenic mouse models. Here we report that IL-33 administration reverses synaptic plasticity impairment and memory deficits in APP/PS1 mice. IL-33 administration reduces soluble Aβ levels and amyloid plaque deposition by promoting the recruitment and Aβ phagocytic activity of microglia; this is mediated by ST2/p38 signaling activation. Furthermore, IL-33 injection modulates the innate immune response by polarizing microglia/macrophages toward an antiinflammatory phenotype and reducing the expression of proinflammatory genes, including IL-1β, IL-6, and NLRP3, in the cortices of APP/PS1 mice. Collectively, our results demonstrate a potential therapeutic role for IL-33 in AD.
“IL-33 ameliorates Alzheimer’s disease-like pathology and cognitive decline” by Amy K. Y. Fu, Kwok-Wang Hung, Michael Y. F. Yuen, Xiaopu Zhou, Deejay S. Y. Mak, Ivy C. W. Chan, Tom H. Cheung, Baorong Zhang, Wing-Yu Fu, Foo Y. Liew, and Nancy Y. Ip in PNAS. Published online May 10 2016 doi:10.1073/pnas.1604032113






