Study finds that arteries adapt to oxidative stress caused by aging.
Although the causes of many age-related diseases remain unknown, oxidative stress is thought to be the main culprit. Oxidative stress has been linked to cardiovascular and neurodegenerative diseases including diabetes, hypertension and age-related cancers. However, researchers at the University of Missouri recently found that aging actually offered significant protection against oxidative stress. These findings suggest that aging may trigger an adaptive response to counteract the effects of oxidative stress on blood vessels.
“Molecules known as reactive oxygen species, or ROS, play an important role in regulating cellular function,” said Steven Segal, a professor of medical pharmacology and physiology at the MU School of Medicine and senior author of the study. “However, the overproduction of ROS can help create a condition referred to as oxidative stress, which can alter the function of cells and interfere with their growth and reproduction.”
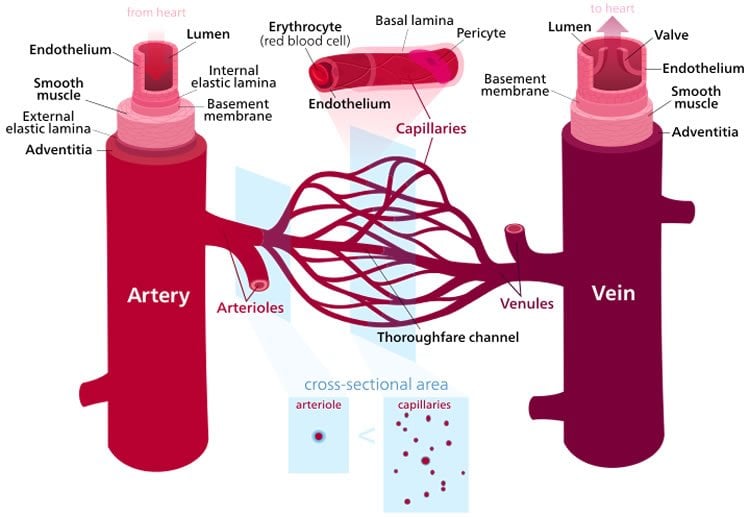
To understand the effects of aging on the function of blood vessels when they are exposed to oxidative stress, Segal’s team studied the inner lining, or endothelium, of small resistance arteries. Resistance arteries are important to cardiovascular function because they regulate both the amount of blood flow into tissues and systemic blood pressure.
“We studied the endothelium from resistance arteries of male mice at 4 months and 24 months of age, which correspond to humans in their early 20s and mid-60s,” Segal said. “We first studied the endothelium under resting conditions and in the absence of oxidative stress. We then simulated oxidative stress by adding hydrogen peroxide. When oxidative stress was induced for 20 minutes, the endothelial cells of the younger mice had abnormal increases in calcium when compared to the endothelial cells of the older mice. This finding is important because when calcium gets too high, cells can be severely damaged.”
When oxidative stress was extended to 60 minutes, Segal’s team found that the death of endothelial cells in the younger mice was seven times greater than those from the older mice. These findings indicated that with advancing age, the endothelium had adapted to preserve cellular integrity when confronted with oxidative stress.
“The most surprising thing we found is that the endothelium was much less perturbed by oxidative stress during advanced age when compared to younger age,” Segal said. “This finding contrasts with the generally held belief that the functional integrity of the endothelium is compromised as we age. Our study suggests that blood vessels adapt during the aging process to regulate ROS and minimize cell death when subjected to an abrupt increase in oxidative stress. This adaptation helps to ensure that the arteries of older individuals can still do their jobs.”
“Although more studies are needed to identify the mechanism by which the endothelium adapts to advanced age, our study provides evidence that the natural tendency of the body is to adapt to oxidative stress during healthy aging,” Segal said.
Funding: Funding for the study was provided by the National Institutes of Health (R37-HL041026, R01-HL086483, F32-HL107050, F32-HL118836, K99-AG047198 and K01-AG041208). The content of the article is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Source: Jeffrey Hoelscher – University of Missouri
Image Credit: The image is credited to Kelvinsong and is licensed CC BY-SA 3.0
Original Research: Full open access research for “Advanced age protects microvascular endothelium from aberrant Ca2+ influx and cell death induced by hydrogen peroxide” by Boerman, Erik J. Behringer, Rebecca L. Shaw, Timothy L. Domeier and Steven S. Segal in Journal of Physiology. Published online May 1 2015 doi:10.1113/JP270169
Abstract
Advanced age protects microvascular endothelium from aberrant Ca2+ influx and cell death induced by hydrogen peroxide
Key points
- Calcium signalling in endothelial cells of resistance arteries is integral to blood flow regulation. Oxidative stress and endothelial dysfunction can prevail during advanced age and we questioned how calcium signalling may be affected.
- Intact endothelium was freshly isolated from superior epigastric arteries of Young (∼4 months) and Old (∼24 months) male C57BL/6 mice. Under resting conditions, with no difference in intracellular calcium levels, hydrogen peroxide (H2O2) availability was ∼1/3 greater in endothelium of Old mice while vascular catalase activity was reduced by nearly half.
- Compared to Old, imposing oxidative stress (200 μm H2O2) for 20 min increased intracellular calcium to 4-fold greater levels in endothelium of Young in conjunction with twice the calcium influx. Prolonged (60 min) exposure to H2O2 induced 7-fold greater cell death in endothelium of Young.
- Microvascular adaptation to advanced age may protect endothelial cells during elevated oxidative stress to preserve functional viability of the intima.
Abstract
Endothelial cell Ca2+ signalling is integral to blood flow control in the resistance vasculature yet little is known of how its regulation may be affected by advancing age. We tested the hypothesis that advanced age protects microvascular endothelium by attenuating aberrant Ca2+ signalling during oxidative stress. Intact endothelial tubes (width, ∼60 μm; length, ∼1000 μm) were isolated from superior epigastric arteries of Young (3–4 months) and Old (24–26 months) male C57BL/6 mice and loaded with Fura-2 dye to monitor [Ca2+]i. At rest there was no difference in [Ca2+]i between age groups. Compared to Young, the [Ca2+]i response to maximal stimulation with acetylcholine (3 μm, 2 min) was ∼25% greater in Old, confirming signalling integrity with advanced age. Basal H2O2 availability was ∼33% greater in Old while vascular catalase activity was reduced by half. Transient exposure to elevated H2O2 (200 μm, 20 min) progressively increased [Ca2+]i to ∼4-fold greater levels in endothelium of Young versus Old. With no difference between age groups at rest, Mn2+ quench of Fura-2 fluorescence revealed 2-fold greater Ca2+ influx in Young during elevated H2O2; this effect was attenuated by ∼75% using ruthenium red (5 μm) as a broad-spectrum inhibitor of transient receptor potential channels. Prolonged exposure to H2O2 (200 μm, 60 min) induced ∼7-fold greater cell death in endothelium of Young versus Old. Thus, microvascular endothelium can adapt to advanced age by reducing Ca2+ influx during elevated oxidative stress. Protection from cell death during oxidative stress will sustain endothelial integrity during ageing.
“Advanced age protects microvascular endothelium from aberrant Ca2+ influx and cell death induced by hydrogen peroxide” by Boerman, Erik J. Behringer, Rebecca L. Shaw, Timothy L. Domeier and Steven S. Segal in Journal of Physiology. Published online May 1 2015 doi:10.1113/JP270169