Summary: Researchers have identified two groups of serotonin neurons that may help to suppress aggression in mice.
Source: Harvard.
How does a small cluster of fairly homogeneous brain cells that make the neurotransmitter serotonin regulate so many functions, from breathing to mood to appetite?
The answer is that they’re not so homogeneous after all. Over the past few years, researchers, including Harvard Medical School geneticist Susan Dymecki, have begun uncovering distinct subtypes of serotonin neurons that appear to have specific responsibilities.
Now, Dymecki and colleagues have identified two groups of serotonin neurons that help suppress aggression in male mice.
The discovery refines scientists’ understanding of the complex neural circuitry underlying aggression and reveals a concrete connection between dopamine-producing neurons and the two specific subtypes of serotonin neurons in the mouse brain.
“These particular serotonin neurons are sensitive to dopamine in distinct and very specific ways,” said Dymecki, senior author of the study published Nov. 15 in Cell Reports.
“There has been evidence of a general interplay between serotonin and dopamine,” she said, “but here we’ve linked the two in a specific group of cells involved in modulating a specific behavior.”
The authors propose that dopamine escalates aggression through a double whammy: both on its own, as has been shown by decades of research, and by suppressing the serotonin neurons that normally soothe aggression, according to the new study.
“A lot of times, you study neurotransmitters like dopamine or serotonin or norepinephrine but you don’t necessarily understand how they’re all working together,” said Tedi Asher, a graduate student in the Dymecki lab and co-first author of the study. “Our work begins to make connections between these systems.”
“It’s particularly exciting here,” Asher added, “where dopamine is thought to increase aggression and serotonin is thought to decrease it, to see that those two systems are talking to each other.”
Although the research was limited to mice, Dymecki believes her team’s observations will also hold true for humans, in part because the relevant area of the hindbrain is similar across mammals.
If confirmed in humans, the findings could lead to more precise treatments for the excess aggression that can arise in conditions such as schizophrenia, intermittent explosive disorder, autism and dementia.
Soothing serotonin
Serotonin is one of many neurotransmitters known to affect aggressive behavior in people and animals. Recent research has linked low serotonin with a higher likelihood of pathological aggression, and doctors sometimes prescribe selective serotonin reuptake inhibitors, or SSRIs, to boost serotonin levels in patients with impulsive aggression.
However, SSRIs are blunt instruments that strike all serotonin neurons. Trying to adjust one serotonin-regulated behavior can affect others, including breathing rate, mood, sex drive and appetite.
Dymecki realized that if she could find out whether only some of the neurons influence aggression, then it might be possible to develop treatments that target those cells alone and reduce unwanted side effects.
Dymecki’s team began by silencing—that is, blocking neurotransmitter release from—the majority of serotonin neurons in a group of lab mice. As expected, those mice became more aggressive, biting, threatening and rattling their tails at other mice more often than did mice with intact serotonin systems.
In earlier studies, Dymecki and others showed that serotonin neurons can be subdivided based on patterns of genes that are turned on or off. Building on that work, the team took a new group of mice and silenced many subtypes one at a time, watching for any that significantly changed the rodents’ aggression levels.
They found two: Drd1a/Pet1 neurons and Drd2/Pet1 neurons.
Dopamine surprise
Mice in which either neuron subtype was silenced were more aggressive. Silencing other subtypes of serotonergic neurons had no effect.
What makes those two special? For one thing, they have dopamine receptors on their surfaces—hence the “Dr” that begins their names. Drd1a/Pet1 cells are studded with dopamine receptor type 1, Drd2/Pet1 cells with dopamine receptor type 2.
Dopamine has a range of roles in the body, from motor control to reward motivation. The researchers discovered that dopamine suppressed Drd2/Pet1 neurons’ ability to fire. Muffling the same neurons using genetic tools similarly dampened aggression in the mice.
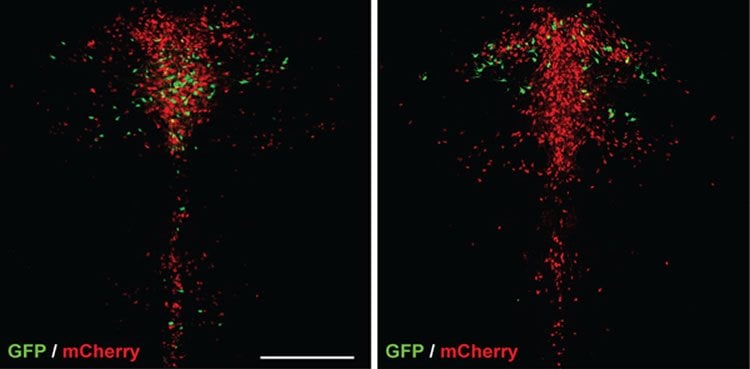
The researchers also found that the majority of serotonin cells nestled right alongside Drd2/Pet1 neurons respond to dopamine in the opposite way, ramping up their activity. Dymecki’s team didn’t detect any dopamine receptors on their surfaces, leaving it a mystery what those cells are doing and how dopamine acts on them.
Further experiments revealed that Drd2/Pet1 cells’ dopamine receptors activate during adolescence. By contrast, Drd1a/Pet1 cells express their dopamine receptors before and briefly after birth.
More to puzzle out
Both serotonin neuron subtypes differed from their brethren in other significant and sometimes unexpected ways.
It made sense to the researchers when they found that Drd1a/Pet1 cells communicate with regions of the brain known to influence aggression, but they were surprised to discover that the Drd2/Pet1 cells connect to regions involved in sensory processing.
“These differences in circuitry may indicate distinct means of impacting the same behavior: aggression,” said Asher.
It’s not yet clear why the mouse brain contains multiple types of dopamine-responsive serotonin neurons, each active at different stages of development. Dymecki’s group intends to find out.
The present findings demonstrate that “a small population of serotonin neurons can have a profound effect on social interaction,” said Dymecki. “We have a precedent now to deconstruct the serotonin dependency of, and the circuitry underlying, distinct aspects of social behavior.”
The other co-first author of the paper is Vera Niederkofler, head of cell culture at the biotechnology company QPS Austria GmbH in Grambach, Austria, who completed the work while a postdoctoral researcher in the Dymecki lab.
Additional collaborators were from HMS, Tufts University, the Perelman School of Medicine at the University of Pennsylvania and Children’s Hospital of Philadelphia.
Funding: This study was funded by the National Institutes of Health (grants AA021622, AA013983, 5R21DA023643-02, R01DA034022 and R21MH083613), the Swiss National Science Foundation and the G.V.R. Khodadad Research Fund at HMS, which was established in 2012 to support research into the neurogenetics of aggressive behavior and excessive selfishness.
Source: Stephanie Dutchen – Harvard
Image Source: NeuroscienceNews.com image is credited to Dymecki lab.
Original Research: Full open access research for “Identification of Serotonergic Neuronal Modules that Affect Aggressive Behavior” by Vera Niederkofler, Tedi E. Asher, Benjamin W. Okaty, Benjamin D. Rood, Ankita Narayan, Lara S. Hwa, Sheryl G. Beck, Klaus A. Miczek, and Susan M. Dymeckin Cell Reports. Published online October 17 2016 doi:10.1016/j.celrep.2016.10.063
[cbtabs][cbtab title=”MLA”]Harvard “Fight Club: Specific Types of Serotonin Neurons Modulate Aggression.” NeuroscienceNews. NeuroscienceNews, 16 November 2016.
<https://neurosciencenews.com/aggression-serotonin-neuromodulation-5530/>.[/cbtab][cbtab title=”APA”]Harvard (2016, November 16). Fight Club: Specific Types of Serotonin Neurons Modulate Aggression. NeuroscienceNew. Retrieved November 16, 2016 from https://neurosciencenews.com/aggression-serotonin-neuromodulation-5530/[/cbtab][cbtab title=”Chicago”]Harvard “Fight Club: Specific Types of Serotonin Neurons Modulate Aggression.” https://neurosciencenews.com/aggression-serotonin-neuromodulation-5530/ (accessed November 16, 2016).[/cbtab][/cbtabs]
Abstract
Identification of Serotonergic Neuronal Modules that Affect Aggressive Behavior
Highlights
•Silencing serotonin (5-HT) neurons increases mouse inter-male aggression
•Aggression effects map to Drd1a/Pet1 and Drd2/Pet1 subtypes of 5-HT neurons
•DA regulates 5-HT neurons, inhibiting a subset via Drd2
•Axonal bouton mapping identifies neural targets of aggression-modulating subtypes
Summary
Escalated aggression can have devastating societal consequences, yet underlying neurobiological mechanisms are poorly understood. Here, we show significantly increased inter-male mouse aggression when neurotransmission is constitutively blocked from either of two subsets of serotonergic, Pet1+ neurons: one identified by dopamine receptor D1(Drd1a)::cre-driven activity perinatally, and the other by Drd2::cre from pre-adolescence onward. Blocking neurotransmission from other Pet1+ neuron subsets of similar size and/or overlapping anatomical domains had no effect on aggression compared with controls, suggesting subtype-specific serotonergic neuron influences on aggression. Using established and novel intersectional genetic tools, we further characterized these subtypes across multiple parameters, showing both overlapping and distinct features in axonal projection targets, gene expression, electrophysiological properties, and effects on non-aggressive behaviors. Notably, Drd2::cre marked 5-HT neurons exhibited D2-dependent inhibitory responses to dopamine in slices, suggesting direct and specific interplay between inhibitory dopaminergic signaling and a serotonergic subpopulation. Thus, we identify specific serotonergic modules that shape aggression.
“Identification of Serotonergic Neuronal Modules that Affect Aggressive Behavior” by Vera Niederkofler, Tedi E. Asher, Benjamin W. Okaty, Benjamin D. Rood, Ankita Narayan, Lara S. Hwa, Sheryl G. Beck, Klaus A. Miczek, and Susan M. Dymeckin Cell Reports. Published online October 17 2016 doi:10.1016/j.celrep.2016.10.063






