Summary: A molecular process caused by the TREM2 gene mutation in the brain’s microglia increases the risk of Alzheimer’s disease, a new study reports.
Source: UC Irvine
The molecular processes caused by a TREM2 (Triggering Receptor Expressed on Myeloid Cells 2) gene mutation in the brain’s microglia immune cells can increase the risk of Alzheimer’s disease, according to a recent study led by researchers at the University of California, Irvine.
While many immune cell genes have been associated with Alzheimer’s, the odds are increased two to three times by mutations in TREM2. However, the processes by which these mutations change the function of microglia cells have not been identified until now.
The study, titled “TREM2 regulates purinergic receptor-mediated calcium signaling and motility in human iPSC-derived microglia” was recently published online in eLife.
A critical function of microglia is restoring brain health. Building on their earlier work showing microglia that lack TREM2 have problems moving toward sites of damage in the brain, the research team discovered that this lack of motility is associated with increased levels of P2RY12 and P2RY13 receptors on the microglia.
When these receptors detect purinergic molecular danger signals released from nearby damaged cells, they rapidly increase internal messenger calcium within the microglia. The microglia containing higher calcium levels don’t turn as frequently and so can’t direct themselves to the sites of injury.
“Our study shows that problems arise from having too much signaling between P2RY12 and P2RY13 receptors. We were able to return the microglia immune cells back to their normal function by blocking these receptors.
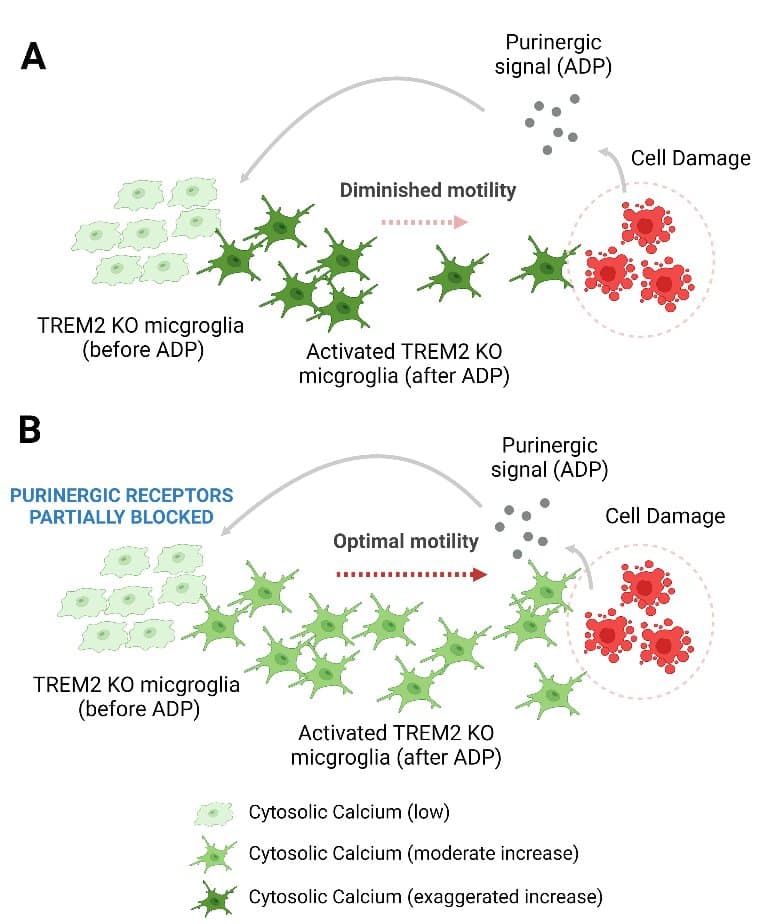
“This suggests that Alzheimer’s patients who have mutations in the TREM2 protein would benefit from inhibition of these receptors or related signaling molecules. Manipulating calcium using drugs could be another way to modulate microglia behavior to fight disease,” said Michael D. Cahalan, Ph.D., Physiology & Biophysics distinguished professor and chair at the UCI School of Medicine, and co-corresponding author.
The team used a culture model of microglia derived from human induced pluripotent stem cells from blood to study the lack of TREM2. A special calcium probe called Salsa6f was engineered into those cells, which allowed calcium to be measured while recording motility characteristics in real time.
“Alzheimer’s disease is the most common cause of dementia and currently has no cure,” Cahalan said. “Studies of DNA from people with Alzheimer’s surprisingly revealed that changes to genes in microglia can have a great impact on whether or not someone gets the disease. Understanding the processes of how these mutations change immune cell function will allow us to develop targeted therapies.”
UCI post doctoral scholar Amit Jairaman, and graduate student Amanda McQuade, who is now a postdoctoral fellow at UCSF, are co-first authors of the study.
About this genetics and Alzheimer’s disease research news
Author: Press Office
Source: UC Irvine
Contact: Press Office – UC Irvine
Image: The image is credited to School of Medicine / UCI
Original Research: Open access.
“TREM2 regulates purinergic receptor-mediated calcium signaling and motility in human iPSC-derived microglia” by Murali Prakriya et al. eLife
Abstract
TREM2 regulates purinergic receptor-mediated calcium signaling and motility in human iPSC-derived microglia
The membrane protein TREM2 (Triggering Receptor Expressed on Myeloid cells 2) regulates key microglial functions including phagocytosis and chemotaxis. Loss-of-function variants of TREM2 are associated with increased risk of Alzheimer’s disease (AD).
Because abnormalities in Ca2+ signaling have been observed in several AD models, we investigated TREM2 regulation of Ca2+ signaling in human induced pluripotent stem cell-derived microglia (iPSC-microglia) with genetic deletion of TREM2. We found that iPSC-microglia lacking TREM2 (TREM2 KO) show exaggerated Ca2+ signals in response to purinergic agonists, such as ADP, that shape microglial injury responses.
This ADP hypersensitivity, driven by increased expression of P2Y12 and P2Y13 receptors, results in greater release of Ca2+ from the endoplasmic reticulum stores, which triggers sustained Ca2+ influx through Orai channels and alters cell motility in TREM2 KO microglia. Using iPSC-microglia expressing the genetically encoded Ca2+ probe, Salsa6f, we found that cytosolic Ca2+ tunes motility to a greater extent in TREM2 KO microglia.
Despite showing greater overall displacement, TREM2 KO microglia exhibit reduced directional chemotaxis along ADP gradients. Accordingly, the chemotactic defect in TREM2 KO microglia was rescued by reducing cytosolic Ca2+ using a P2Y12 receptor antagonist.
Our results show that loss of TREM2 confers a defect in microglial Ca2+ response to purinergic signals, suggesting a window of Ca2+ signaling for optimal microglial motility.






