Summary: Glucose takes longer to get into the nucleus accumbens of obesity-prone rat models. Researchers also discovered excess levels of glutamate in obesity-prone rats, implying a deficiency in the neurotransmitter recycling process normally maintained by astrocytes.
Source: University of Michigan
On a diet? Perhaps you’re avoiding sweets or carbs altogether or curbing late-night munchies. These are examples of behavior modifications, and when it comes to food, avoiding those diet triggers can be pretty hard to do.
To understand what drives people to overeat, scientists are looking more closely at a brain structure involved in motivation, called the nucleus accumbens. This small region drives reward-seeking behaviors underlying the pursuit of sex, recreational drugs like nicotine and alcohol, and food.
“These brain motivation centers evolved to help us survive; finding food and having sex are essential to the survival of an individual and of a species,” said Carrie Ferrario, Ph.D., associate professor in the Department of Pharmacology at U-M Medical School.
“What was advantageous when food was hard to find has become a disadvantage and unhealthy in the current food dense environment. This is compounded by the over-abundance of over-processed, low nutrition foods that may satisfy our taste but leave our bodies unnourished.
“People don’t tend to find it difficult to turn down an extra serving of broccoli, but just one more french-fry or making room for a bit of chocolate dessert… that’s a different story. The real challenge is overcoming these urges and changing our behavior when it comes to food,” Ferrario added.
Given the immense toll obesity takes on virtually all body systems, Ferrario, Peter Vollbrecht, Ph.D., of Western Michigan University, and their colleagues are using rat models to understand potential brain differences between animals who are prone to overeating and obesity and those who are not.
Previous research from Ferrario’s lab pinpointed differences in the nucleus accumbens in obesity-prone and obesity-resistant rats. Their latest study, published in the Journal of Neurochemistry, tracked what was happening in real time in the brain when these animals were presented with glucose, a type of sugar, labeled with a tracer. The tracer allowed the researchers to measure this new sugar in the brain.
Sugar is the brain’s main fuel source, and once there, the molecule is broken down and used to create new molecules such as glutamine, glutamate, and GABA, each with an important role in influencing the activation of neurons in the brain and nervous system.
“Glucose that is consumed gets broken down and then its carbons get incorporated into neurotransmitters. We see those labeled carbons showing up in those molecules—glutamate, glutamine, and GABA—over time,” explained Vollbrecht.
They found that glucose was taking longer to get into the nucleus accumbens of obesity-prone animals.
Furthermore, when measuring the concentration of the glutamate, glutamine, and GABA, they discovered excess levels of glutamate, an excitatory neurotransmitter. This, said the team, implied a defect in a neurotransmitter recycling process, typically maintained in the nervous system by star-shaped cells called astrocytes.
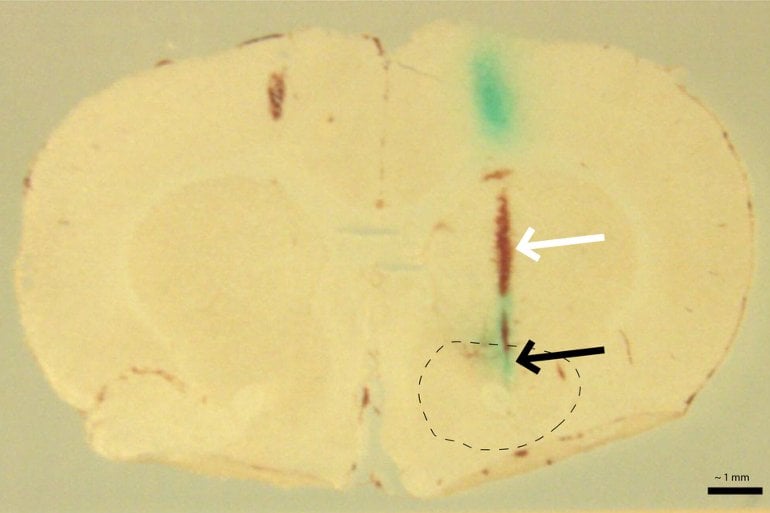
Normally, astrocytes will pull glutamate out of the space between neurons, called the synapse, convert it into glutamine, and then shuttle it back to cells that produce GABA or glutamate. This sequence is crucial for turning neurons off and on. “The findings suggest that we’re getting too much glutamate and it’s not being taken out of the synapse,” said Vollbrecht.
Ferrario added, “The balance between glutamate and GABA (the main inhibitory transmitter) is really important for brain function and will influence activity of the neurons in the nucleus accumbens.”
This balance, and therefore brain activity, is different in obesity-prone vs. obesity-resistant rats.
The fact that these rats are either prone to obesity or not is important for disentangling cause and effect, says Vollbrecht. “It allows us to remove diet as one of the variables.”
The team hopes to next study the role of inflammation in the development of obesity, and how differences in brain function contribute to susceptibility and resistance to obesity.
Other authors on the paper include Kathryn M. Nesbitt, Victoria M. Addis, Keenan M. Boulnemour, Daniel A. Micheli, Kendall B. Smith, Darleen A. Sandoval, and Robert T. Kennedy.
About this neuroscience research news
Author: Press Office
Source: University of Michigan
Contact: Press Office – University of Michigan
Image: The image is credited to the researchers
Original Research: Open access.
“Differential regulation of nucleus accumbens glutamate and GABA in obesity‐prone and obesity‐resistant rats” by Peter J. Vollbrecht et al. Journal of Neurochemistry
Abstract
Differential regulation of nucleus accumbens glutamate and GABA in obesity‐prone and obesity‐resistant rats
Obesity is one of the leading health concerns in the United States. Studies from human and rodent models suggest that inherent differences in the function of brain motivation centers, including the nucleus accumbens (NAc), contribute to overeating and thus obesity.
For example, there are basal enhancements in the excitability of NAc GABAergic medium spiny neurons (MSN) and reductions in basal expression of AMPA-type glutamate receptors in obesity-prone vs obesity-resistant rats. However, very little is known about the regulation of extracellular glutamate and GABA within the NAc of these models.
Here we gave obesity-prone and obesity-resistant rats stable isotope-labeled glucose (13C6-glucose) and used liquid chromatography mass spectrometry (LC–MS) analysis of NAc dialysate to examine the real-time incorporation of 13C6-glucose into glutamate, glutamine, and GABA.
This novel approach allowed us to identify differences in glucose utilization for neurotransmitter production between these selectively bred lines. We found that voluntarily ingested or gastrically infused 13C6-glucose rapidly enters the NAc and is incorporated into 13C2-glutamine, 13C2-glutamate, and 13C2-GABA in both groups within minutes.
However, the magnitude of increases in NAc 13C2-glutamine and 13C2-GABA were lower in obesity-prone than in obesity-resistant rats, while basal levels of glutamate were elevated.
This suggested that there may be differences in the astrocytic regulation of these analytes. Thus, we next examined NAc glutamine synthetase, GAD67, and GLT-1 protein expression. Consistent with reduced 13C2-glutamine and 13C2-GABA, NAc glutamine synthetase and GLT-1 protein expression were reduced in obesity-prone vs obesity-resistant groups.
Taken together, these data show that NAc glucose utilization differs dramatically between obesity-prone and obesity-resistant rats, favoring glutamate over GABA production in obesity-prone rats and that reductions in NAc astrocytic recycling of glutamate contribute to these differences.
These data are discussed in light of established differences in NAc function between these models and the role of the NAc in feeding behavior.