Summary: Researchers may have solved one of the great mysteries of neuroscience; namely, why neurons in the temporal lobe are the first to die in Alzheimer’s disease, and why dopaminergic neurons are damaged first in Parkinson’s.
Source: University of Virginia Health System.
One of the great mysteries of neuroscience may finally have an answer: Scientists at the University of Virginia School of Medicine have identified a potential explanation for the mysterious death of specific brain cells seen in Alzheimer’s, Parkinson’s and other neurodegenerative diseases.
The new research suggests that the cells may die because of naturally occurring gene variation in brain cells that were, until recently, assumed to be genetically identical. This variation – called “somatic mosaicism” – could explain why neurons in the temporal lobe are the first to die in Alzheimer’s, for example, and why dopaminergic neurons are the first to die in Parkinson’s.
“This has been a big open question in neuroscience, particularly in various neurodegenerative diseases,” said neuroscientist Michael McConnell, PhD, of UVA’s Center for Brain Immunology and Glia (BIG). “What is this selective vulnerability? What underlies it? And so now, with our work, the hypotheses moving forward are that it could be that different regions of the brain actually have a different garden of these [variations] in young individuals and that sets up different regions for decline later in life.”
A Most Unexpected Outcome
The finding emerged unexpectedly from McConnell’s investigations into schizophrenia. It was in that context that he and his collaborators first discovered the unexpected variation in the genetic makeup of individual brain cells. That discovery may help explain not just schizophrenia but depression, bipolar disorder, autism and other conditions.
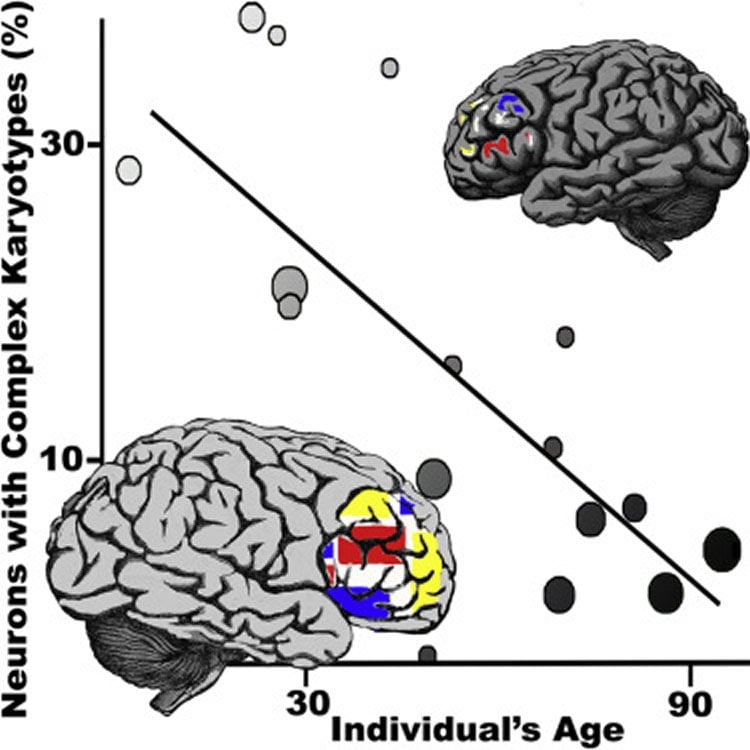
Continuing his investigations, McConnell expected that this mosaicism would increase with age – that mutations would accumulate over time. What he and his collaborators at Johns Hopkins found is exactly the opposite: Younger people had the most mosaicism and older people had the least.
“We wound up building an atlas that contained neurons from 15 individuals. None of these individuals had disease,” said McConnell, of UVA’s Department of Biochemistry and Molecular Genetics and UVA’s Department of Neuroscience. “They ranged in age from less than a year to 94 years, and it showed a perfect correlation – a perfect anti-correlation – with age.”
Based on the finding, McConnell believes that the neurons with significant genetic variation, known as CNV neurons, may be the most vulnerable to dying. And that could explain the idiosyncratic death of specific neurons in different neurodegenerative diseases. People with the most CNV neurons in the temporal lobe, for example, might be likely to develop Alzheimer’s.
More work needs to be done to fully understand what’s occurring, McConnell said. So far, he has only looked at neurons in the frontal cortex of the brain, and his studies are limited by the fact that neurons can be examined only after death, so it can be hard to make direct comparisons. But he is excited to expand the scope of his research.
“Because I’m collaborating with the Lieber Institute and they have this fantastic brain bank, now I can look at individuals’ frontal cortex [for the schizophrenia research] and I can look at the temporal lobe in those same individuals,” McConnell said. “So now I can really start to map things out more carefully, building an atlas of different brain regions from many individuals.”
That research could greatly advance our understanding of both neurodegenerative diseases and the cognitive decline that besets us with age, potentially leading to new treatments.
“What’s really interesting about mosaicism is that it is fundamentally tweaking our assumptions about what nature is, because we’ve kind of always assumed that every cell in any given individual had the same genome, the same DNA in every cell,” McConnell said. “And now we’re showing that it’s different and what that might mean.”
Source: Josh Barney – University of Virginia Health System
Publisher: Organized by NeuroscienceNews.com.
Image Source: NeuroscienceNews.com image is credited to McConnell et al/Cell Reports.
Original Research: Open access research for “Neurons with Complex Karyotypes Are Rare in Aged Human Neocortex” by William D. Chronister, Ian E. Burbulis, Margaret B. Wierman, Matthew J. Wolpert, Mark F. Haakenson, Aiden C.B. Smith, Joel E. Kleinman, Thomas M. Hyde, Daniel R. Weinberger, Stefan Bekiranov, and Michael J. McConnell in Cell Reports. Published February 15 2019.
doi:10.1016/j.celrep.2018.12.107
[cbtabs][cbtab title=”MLA”]University of Virginia Health System”Brain Discovery Explains A Great Mystery of Alzheimer’s and Parkinson’s.” NeuroscienceNews. NeuroscienceNews, 15 February 2019.
<https://neurosciencenews.com/somatic-mosaicism-alzheimers-10748/>.[/cbtab][cbtab title=”APA”]University of Virginia Health System(2019, February 15). Brain Discovery Explains A Great Mystery of Alzheimer’s and Parkinson’s. NeuroscienceNews. Retrieved February 15, 2019 from https://neurosciencenews.com/somatic-mosaicism-alzheimers-10748/[/cbtab][cbtab title=”Chicago”]University of Virginia Health System”Brain Discovery Explains A Great Mystery of Alzheimer’s and Parkinson’s.” https://neurosciencenews.com/somatic-mosaicism-alzheimers-10748/ (accessed February 15, 2019).[/cbtab][/cbtabs]
Abstract
Neurons with Complex Karyotypes Are Rare in Aged Human Neocortex
A subset of human neocortical neurons harbors complex karyotypes wherein megabase-scale copy-number variants (CNVs) alter allelic diversity. Divergent levels of neurons with complex karyotypes (CNV neurons) are reported in different individuals, yet genome-wide and familial studies implicitly assume a single brain genome when assessing the genetic risk architecture of neurological disease. We assembled a brain CNV atlas using a robust computational approach applied to a new dataset (>800 neurons from 5 neurotypical individuals) and to published data from 10 additional neurotypical individuals. The atlas reveals that the frequency of neocortical neurons with complex karyotypes varies widely among individuals, but this variability is not readily accounted for by tissue quality or CNV detection approach. Rather, the age of the individual is anti-correlated with CNV neuron frequency. Fewer CNV neurons are observed in aged individuals than in young individuals.






