Summary: Researchers reveal the critical role the p62 gene plays in the selective autophagy of tau oligomers.
Source: National Institutes for Quantum Science and Technology
In order to maintain cellular homeostasis (i.e., a state of equilibrium), cells undergo selective autophagy or self-degradation of unwanted proteins. Autophagy receptors control this process, by mediating the selection of a target protein that is then “cleared.”
Tau proteins—which otherwise play an important role in stabilizing and maintaining the internal organization of neurons in the brain—abnormally accumulate inside neurons in conditions like dementia and Alzheimer’s disease.
This build-up of hyper-phosphorylated tau proteins (or tau oligomers) causes the formation of neurofibrillary tangles (NFTs) and eventual cell death of neurons in the brains of people with dementia, contributing to the disease’s progressive neurodegenerative symptoms.
Now, while tau proteins can be degraded by selective autophagy, the exact mechanism of how this occurs remains a mystery.
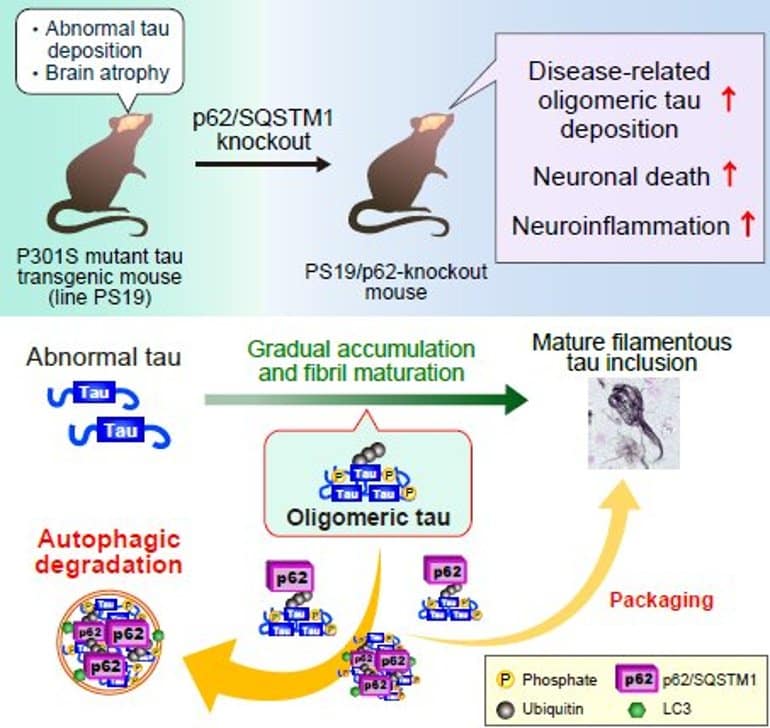
In a recent breakthrough, however, a study done by scientists at the National Institutes for Quantum Science and Technology in Japan proved the critical role played by a certain gene—the p62 gene—in the selective autophagy of tau oligomers. The team included researcher Maiko Ono, and group leader Naruhiko Sahara—both from the Department of Functional Brain Imaging at the National Institutes for Quantum Science and Technology in Japan.
Their paper, published in Aging Cell, was made available online on 5 June 2022.
Previous studies have reported that the abnormal accumulation of the tau proteins may be selectively suppressed by autophagy pathways, through the p62 receptor protein (which is a selective autophagy receptor protein).
Says Maiko Ono, “This protein’s ubiquitin-binding ability helps in the identification of toxic protein aggregates (like tau oligomers), which can then be degraded by cellular processes and organelles.”
This study’s novelty, however, lay in the demonstration of p62’s “neuroprotective” role in a living model, which had never been done before. So, how did the researchers achieve this? They used mouse models of dementia. The p62 gene had been deleted (or knocked out) in one group of these mice, so they did not express p62 receptor proteins.
On studying the brains of these mice using immunostaining and comparative biochemical analyses, an interesting picture was revealed. Neurotoxic tau protein aggregates were found in the hippocampus—the area of the brain associated with memory—and brainstem—the center that coordinates the body’s breathing, heartbeat, blood pressure, and other voluntary processes—of p62 knockout (KO) mice.
When we consider this along with the symptoms of dementia, which include memory loss, confusion, and mood changes, these findings make a lot of sense.
MRI scans revealed that the hippocampus of p62 KO mice was degenerated (atrophied) and inflamed. A postmortem assessment of their brains revealed a greater loss of neurons in their hippocampus.
Further immunofluorescent studies showed that the abnormal tau species aggregates can cause cytotoxicity leading to inflammation and cell death of neurons in p62 KO mice. Oligomeric tau, specifically, accumulated more in the brains of p62 KO mice.
Overall, the findings of this study prove that by eliminating and, hence, preventing the aggregation of oligomeric tau species in the brain, p62 played a neuroprotective role in models of dementia.
At a time when researchers across the word are trying to develop drugs for dementia and other related neurodegenerative disorders, the findings of this study will be of great importance in providing evidence for the accurate targeting of tau oligomers.
The global population of aging humans is increasing each year; hence, the need to develop methods to slow down the onset and progression of various neurodegenerative diseases is also expanding.
This study provides a positive step towards addressing that need.
About this genetics and neuroscience research news
Author: Press Office
Source: National Institutes for Quantum Science and Technology
Contact: Press Office – National Institutes for Quantum Science and Technology
Image: The image is credited to National Institutes for Quantum Science and Technology
Original Research: Open access.
“Central role for p62/SQSTM1 in the elimination of toxic tau species in a mouse model of tauopathy” by Maiko Ono et al. Aging Cell
Abstract
Central role for p62/SQSTM1 in the elimination of toxic tau species in a mouse model of tauopathy
Intracellular accumulation of filamentous tau aggregates with progressive neuronal loss is a common characteristic of tauopathies. Although the neurodegenerative mechanism of tau-associated pathology remains unclear, molecular elements capable of degrading and/or sequestering neurotoxic tau species may suppress neurodegenerative progression.
Here, we provide evidence that p62/SQSTM1, a ubiquitinated cargo receptor for selective autophagy, acts protectively against neuronal death and neuroinflammation provoked by abnormal tau accumulation.
P301S mutant tau transgenic mice (line PS19) exhibited accumulation of neurofibrillary tangles with localization of p62 mostly in the brainstem, but neuronal loss with few neurofibrillary tangles in the hippocampus.
In the hippocampus of PS19 mice, the p62 level was lower compared to the brainstem, and punctate accumulation of phosphorylated tau unaccompanied by co-localization of p62 was observed. In PS19 mice deficient in p62 (PS19/p62-KO), increased accumulation of phosphorylated tau, acceleration of neuronal loss, and exacerbation of neuroinflammation were observed in the hippocampus as compared with PS19 mice. In addition, increase of abnormal tau and neuroinflammation were observed in the brainstem of PS19/p62-KO.
Immunostaining and dot-blot analysis with an antibody selectively recognizing tau dimers and higher-order oligomers revealed that oligomeric tau species in PS19/p62-KO mice were significantly accumulated as compared to PS19 mice, suggesting the requirement of p62 to eliminate disease-related oligomeric tau species.
Our findings indicated that p62 exerts neuroprotection against tau pathologies by eliminating neurotoxic tau species, suggesting that the manipulative p62 and selective autophagy may provide an intrinsic therapy for the treatment of tauopathy.






