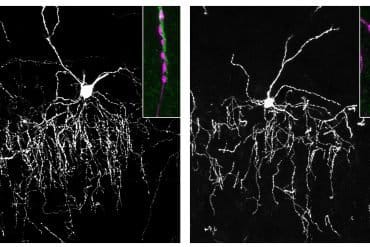
Summary: Researchers have identified a rare type of brain cell that may drive the chronic inflammation and neurodegeneration seen in progressive multiple sclerosis (MS). These cells, called disease-associated radial glia-like (DARG) cells, appear six times more often in patients with progressive MS than in healthy individuals.
DARGs revert to an early developmental state yet also show premature aging, releasing inflammatory signals that accelerate brain cell damage. The discovery could pave the way for targeted treatments that either repair or remove these dysfunctional cells, marking a major step toward halting disease progression.
Key Facts:
- Newly Identified Cell Type: DARGs are radial glia-like cells found in high numbers in progressive MS, linking abnormal development to neurodegeneration.
- Inflammatory Role: These cells release immune signals that induce premature aging in nearby brain cells, worsening inflammation and damage.
- Therapeutic Potential: Targeting or eliminating DARGs could lead to the first disease-modifying treatments for progressive MS.
Source: University of Cambridge
Scientists have identified an unusual type of brain cell that may play a vital role in progressive multiple sclerosis (MS), likely contributing to the persistent inflammation characteristic of the disease.
The discovery, reported today in Neuron, is a significant step towards understanding the complex mechanisms that drive the disease and provides a promising new avenue for research into more effective therapies for this debilitating condition.
MS is a chronic disease in which the immune system mistakenly attacks the brain and spinal cord, disrupting communication between the brain and the body. While many individuals initially experience relapses and remissions, a significant proportion transition to progressive MS, a phase marked by a steady decline in neurological function with limited treatment options.
To model what is happening in the disease, researchers at the University of Cambridge, UK, and National Institute on Aging, US, took skin cells from patients with progressive MS and reprogrammed them into induced neural stem cells (iNSCs), an immature type of cell capable of dividing and differentiating into various types of brain cells.
Using this ‘disease in a dish’ approach, the team observed that a subset of the cultured brain cells was somehow reverting to an earlier developmental stage, transforming into an unusual cell type known as radial glia-like (RG-like) cells.
Notably, these cells were highly specific and appeared approximately six times more frequently in iNSC lines derived from individuals with progressive MS compared to controls. As a result, they were designated as disease-associated RG-like cells (DARGs).
These DARGs exhibit characteristic features of radial glia—specialized cells that serve as scaffolding during brain development and possess the capacity to differentiate into various neural cell types.
Essentially, they function both as structural support and as fundamental building blocks, making them critical for proper brain development. Unexpectedly, DARGs not only revert to an ‘infant’ state but also display hallmark features of premature aging, or senescence.
These newly identified DARGs possess a distinctive epigenetic profile—patterns of chemical modifications that regulate gene activity—although the factors influencing this epigenetic landscape remain unclear.
These modifications contribute to an exaggerated response to interferons, the immune system’s ‘alarm signals,’ which may help explain the high levels of inflammation observed in MS.
Professor Stefano Pluchino from the Department of Clinical Neurosciences at the University of Cambridge, joint senior author, said: “Progressive MS is a truly devastating condition, and effective treatments remain elusive. Our research has revealed a previously unappreciated cellular mechanism that appears central to the chronic inflammation and neurodegeneration driving the progressive phase of the disease.
“Essentially, what we’ve discovered are glial cells that don’t just malfunction – they actively spread damage. They release inflammatory signals that push nearby brain cells to age prematurely, fuelling a toxic environment that accelerates neurodegeneration.”
The team validated their findings by cross-referencing with human data from individuals with progressive MS. By analysing gene expression patterns at the single-cell level—including new data exploring the spatial context of RNA within post-mortem MS brain tissue—they confirmed that DARGs are specifically localised within chronically active lesions, the regions of the brain that sustain the most significant damage.
Importantly, DARGs were found near inflammatory immune cells, supporting their role in orchestrating the damaging inflammatory environment characteristic of progressive MS.
By isolating and studying these disease-driving cells in vitro, the researchers aim to explore their complex interactions with other brain cell types, such as neurons and immune cells. This approach will help to explain the cellular crosstalk that contributes to disease progression in progressive MS, providing deeper insights into underlying pathogenic mechanisms.
Dr Alexandra Nicaise, co-lead author of the study from the Department of Clinical Neurosciences at Cambridge, added: “We’re now working to explore the molecular machinery behind DARGs, and test potential treatments. Our goal is to develop therapies that either correct DARG dysfunction or eliminate them entirely.
“If we’re successful, this could lead to the first truly disease-modifying therapies for progressive MS, offering hope to thousands living with this debilitating condition.”
To date, DARGs have only ever been seen in a handful of diseases, such as glioblastoma and cerebral cavernomas, clusters of abnormal blood vessels. However, this may be because scientists have until now lacked the tools to find them.
Professor Pluchino and colleagues believe their approach is likely to reveal that DARGs play an important role in other forms of neurodegeneration.
Funding: This work received funding from the Medical Research Council, the Wellcome Trust, the National MS Society, FISM – Fondazione Italiana Sclerosi Multipla, the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), the National Institute on Aging, the UK Dementia Research Institute, the Austrian Science Fund FWF, the UK MS Society Centre of Excellence, the Bascule Charitable Trust, and the Ferblanc Foundation.
Key Questions Answered:
A: DARGs (disease-associated radial glia-like cells) are immature, inflammation-promoting brain cells that reappear and age prematurely in progressive MS.
A: They may explain why chronic inflammation persists in progressive MS and could serve as a novel target for therapies aimed at slowing or reversing damage.
A: Scientists reprogrammed patient skin cells into neural stem cells, revealing the unexpected presence and behavior of DARGs in progressive MS samples.
About this neurology and multiple sclerosis research news
Author: Craig Brierley
Source: University of Cambridge
Contact: Craig Brierley – University of Cambridge
Image: The image is credited to Neuroscience News
Original Research: Open access.
“Integrated Multi-Omics Reveals Disease-Associated Radial Glia-like Cells with Epigenetically Dysregulated Interferon Response in Progressive Multiple Sclerosis” by Stefano Pluchino et al. Neuron
Abstract
Integrated Multi-Omics Reveals Disease-Associated Radial Glia-like Cells with Epigenetically Dysregulated Interferon Response in Progressive Multiple Sclerosis
Progressive multiple sclerosis (PMS) involves a persistent, maladaptive inflammatory process with numerous cellular drivers.
We generated induced neural stem cells (iNSCs) from patient fibroblasts through a direct reprogramming protocol that preserved their epigenome, which revealed a PMS-specific hypomethylation of lipid metabolism and interferon (IFN) signaling genes.
Single-cell multi-omics uncovered a novel, disease-associated radial glia-like cell (DARG) subpopulation in PMS cell lines exhibiting senescence and potent IFN responsiveness driven by specific transcription factors.
Functionally, PMS iNSCs induced paracrine senescence and inflammation onto control cells, which was inhibited upon senolytic treatment.
We identified in PMS brains a distinct population of senescent, IFN-responsive DARGs that developmentally aligned with the trajectories of iNSCs in vitro and spatially associated with inflammatory glia in chronically active lesions.
DARGs may sustain smoldering inflammation, unveiling a previously unrecognized cellular axis that could underpin mechanisms in neurodegeneration.
This discovery offers novel insights into disease mechanisms and highlights potential therapeutic targets.