Summary: CSHL researchers document how neuroblasts make their journey through the rostral migratory stream to their target destination in the olfactory bulb.
Source: Cold Spring Harbor Laboratory.
One of the most hopeful discoveries of modern neuroscience is firm proof that the human brain is not static following birth. Rather, it is continually renewing itself, via a process called postnatal neurogenesis – literally, the birth of new neurons. It begins not long after birth and continues into old age. There is some evidence that when people respond to depression treatment, be it a pill or talk therapy, it has something to do with the wiring up of new neurons.
For reasons still not understood, only two parts of the human brain receive replenishments of neurons postnatally. One is a section of a tiny seahorse-shaped structure called the hippocampus, central in memory and learning. The other is the olfactory bulb, located in a small patch of tissue inside the nose, which receives signals from the environment and helps make them intelligible so they can serve as a basis for action – for instance, to recoil from curdled milk or veer from a stinking skunk.
This week in the Journal of Cell Biology, Professor Linda Van Aelst and colleagues at Cold Spring Harbor Laboratory (CSHL) describe for the first time (in mice) how baby neurons – precursors called neuroblasts, generated from a permanent pocket of stem cells in a brain area called the V-SVZ – make an incredible journey from their place of birth through a special tunnel called the RMS to their target destination in the olfactory bulb. They travel as far as 8 mm, “a huge distance, when you consider how tiny the mouse brain is,” Van Aelst says.
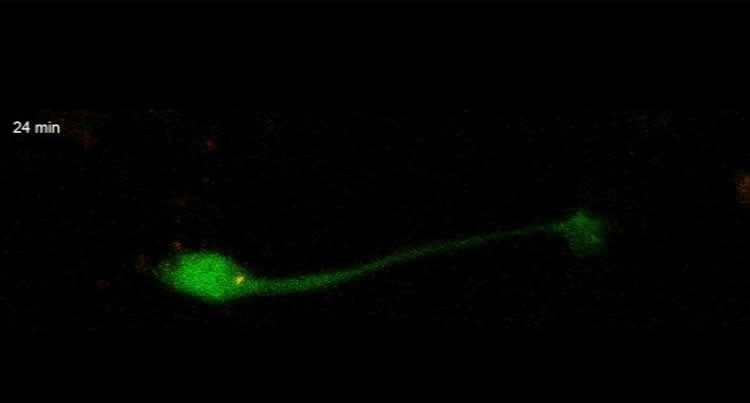
The journey is made possible by two forces, one pulling from the front, the other pushing from behind. A single protein called DOCK7 helps to orchestrate these two steps. Ahead of the newborn neuron’s soma, or cell body, is a threadlike projection called a process. It stretches forward through the tunnel, guided by various signals. At the same time, the cell body, lagging behind, is powered forward by the activation of tiny molecular motors that push it from the rear. Multiple cells migrate together, one virtually on top of another, somewhat in the manner of a group of tiny worms inching forward by morphing the shape of their bodies.
The Takeaway: Neuroscientists have clarified the mechanism used by newly born neurons in mice to migrate to very specific areas in the olfactory bulb. The bulb must be replenished periodically through life to work properly. Knowing how the replenishment works is part of a much broader effort that aims to enhance natural neurogenesis to repair damaged tissue or treat brain disorders.
Funding: National Institutes of Health; Uehara Memorial Foundation
Source: Peter Tarr – Cold Spring Harbor Laboratory
Publisher: Organized by NeuroscienceNews.com.
Image Source: NeuroscienceNews.com image is credited to Van Aelst Lab, CSHL.
Original Research: Abstract for “Dual role for DOCK7 in tangential migration of interneuron precursors in the postnatal forebrain” by Shinichi Nakamuta, Yu-Ting Yang, Chia-Lin Wang, Nicholas B. Gallo, Jia-Ray Yu, Yilin Tai, and Linda Van Aelst in Journal of Cell Biology. Published online October 31 2017 doi:10.1083/jcb.201704157
[cbtabs][cbtab title=”MLA”]Cold Spring Harbor Laboratory “How Newborn Neurons Find Their Proper Place in the Adult Brain.” NeuroscienceNews. NeuroscienceNews, 3 November 2017.
<https://neurosciencenews.com/neurons-movement-adult-7861/>.[/cbtab][cbtab title=”APA”]Cold Spring Harbor Laboratory (2017, November 3). How Newborn Neurons Find Their Proper Place in the Adult Brain. NeuroscienceNews. Retrieved November 3, 2017 from https://neurosciencenews.com/neurons-movement-adult-7861/[/cbtab][cbtab title=”Chicago”]Cold Spring Harbor Laboratory “How Newborn Neurons Find Their Proper Place in the Adult Brain.” https://neurosciencenews.com/neurons-movement-adult-7861/ (accessed November 3, 2017).[/cbtab][/cbtabs]
Abstract
Dual role for DOCK7 in tangential migration of interneuron precursors in the postnatal forebrain
Throughout life, stem cells in the ventricular–subventricular zone generate neuroblasts that migrate via the rostral migratory stream (RMS) to the olfactory bulb, where they differentiate into local interneurons. Although progress has been made toward identifying extracellular factors that guide the migration of these cells, little is known about the intracellular mechanisms that govern the dynamic reshaping of the neuroblasts’ morphology required for their migration along the RMS. In this study, we identify DOCK7, a member of the DOCK180-family, as a molecule essential for tangential neuroblast migration in the postnatal mouse forebrain. DOCK7 regulates the migration of these cells by controlling both leading process (LP) extension and somal translocation via distinct pathways. It controls LP stability/growth via a Rac-dependent pathway, likely by modulating microtubule networks while also regulating F-actin remodeling at the cell rear to promote somal translocation via a previously unrecognized myosin phosphatase–RhoA–interacting protein-dependent pathway. The coordinated action of both pathways is required to ensure efficient neuroblast migration along the RMS.
“Dual role for DOCK7 in tangential migration of interneuron precursors in the postnatal forebrain” by Shinichi Nakamuta, Yu-Ting Yang, Chia-Lin Wang, Nicholas B. Gallo, Jia-Ray Yu, Yilin Tai, and Linda Van Aelst in Journal of Cell Biology. Published online October 31 2017 doi:10.1083/jcb.201704157






