Summary: A drug currently being tested in cancer clinical trials appears to prevent dysfunction in an immune cell signaling pathway associated with Alzheimer’s disease. Blocking the pathway could prevent Alzheimer’s from developing and slow the progression of symptoms for those who already have the disease.
Source: Weill Cornell University
A gene mutation linked to Alzheimer’s disease alters a signaling pathway in certain immune cells of individuals with the disease, according to a new study by scientists at Weill Cornell Medicine.
The team also found that blocking the pathway—with a drug that’s currently being tested in cancer clinical trials—protects against many features of the condition in a preclinical model.
The results could lead to new strategies to block the development of Alzheimer’s disease or slow its progression.
The study, published Dec. 1 in Science Translational Medicine, focused on microglia, immune cells of the central nervous system that are the first to respond when something goes wrong in the brain.
Studies have identified many genetic variants linked to Alzheimer’s disease that are highly expressed in microglia, providing compelling evidence that alterations within these cells may play a role in the disease’s onset and progression.
“Microglia are guardians of the brain under healthy conditions, but can turn detrimental in disease conditions. Our goal is to identify how they become toxic and contribute to Alzheimer’s disease pathogenesis and whether we can identify immune modulators to reverse the toxicity without diminishing their normal protective function,” said senior author Dr. Li Gan, director of the Helen and Robert Appel Alzheimer’s Disease Research Institute and the Burton P. and Judith B. Resnick Distinguished Professor in Neurodegenerative Diseases in the Feil Family Brain and Mind Research Institute at Weill Cornell Medicine.
Alzheimer’s disease is the most prevalent neurodegenerative disease in aging, affecting approximately 46 million people worldwide. Theories point to a number of potential causes, including age-related changes in the brain, along with genetic, environmental, and lifestyle factors. These lead to the accumulation of toxic proteins in the brain—and according to recent evidence, immune system changes—that result in loss of neurons and their connections.
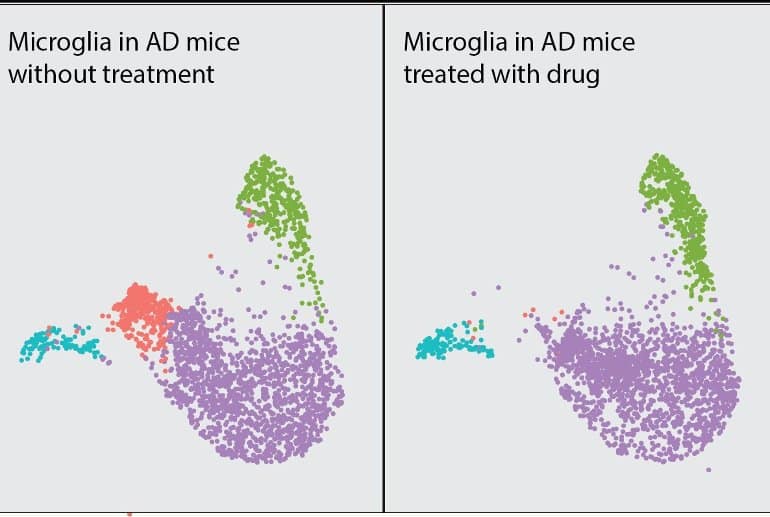
To examine how the brain’s immune cells may contribute to Alzheimer’s disease, Dr. Gan and her colleagues first established the molecule fingerprint of individual microglia in the brains of patients with Alzheimer’s disease who carry a mutation in the TREM2 gene that markedly elevates individual’s risk for developing Alzheimer’s disease.
TREM2 is a receptor mainly expressed by microglia in the brain, and among other functions, it signals through an enzyme named AKT to modulate inflammation and metabolism.
The team then established a mouse model by combining two strains; one that carries the AD-linked mutation in the TREM2 gene and another that exhibits Tau aggregates, one of the major pathological hallmarks in Alzheimer brains.
Both patients and mice with the mutation demonstrated memory-related deficits, and their microglia expressed high levels of inflammatory molecules and exhibited an overactive AKT signaling pathway. In the mice, inhibiting AKT with a drug called MK-2206 reversed the inflammatory properties of microglia and protected against synaptic toxicity—a type of damage to the brain’s neurons that is a hallmark of Alzheimer’s disease.
Importantly, because AKT signaling also contributes to the pathogenesis of many types of cancer, MK-2206 is currently being evaluated in multiple cancer clinical trials. Therefore, the safety of the drug is already under investigation.
“We identified a small molecule compound that has been tested in patients with cancer, readily enters the brain, potently modulates the brain’s immune responses, and protects against synaptic loss in animal models of Alzheimer’s disease,” Dr. Gan said. “Our findings support further study of this compound as a potential therapy for Alzheimer’s disease.”
About this Alzheimer’s disease research news
Author: Press Office
Source: Weill Cornell University
Contact: Press Office – Weill Cornell University
Image: The image is credited to Dr. Li Gan.
Original Research: Closed access.
“AD-linked R47H-TREM2 mutation induces disease-enhancing microglial states via AKT hyperactivation” by Li Gan et al. Science Translational Medicine
Abstract
AD-linked R47H-TREM2 mutation induces disease-enhancing microglial states via AKT hyperactivation
The hemizygous R47H variant of triggering receptor expressed on myeloid cells 2 (TREM2), a microglia-specific gene in the brain, increases risk for late-onset Alzheimer’s disease (AD).
Using transcriptomic analysis of single nuclei from brain tissues of patients with AD carrying the R47H mutation or the common variant (CV)–TREM2, we found that R47H-associated microglial subpopulations had enhanced inflammatory signatures reminiscent of previously identified disease-associated microglia (DAM) and hyperactivation of AKT, one of the signaling pathways downstream of TREM2.
We established a tauopathy mouse model with heterozygous knock-in of the human TREM2 with the R47H mutation or CV and found that R47H induced and exacerbated TAU-mediated spatial memory deficits in female mice. Single-cell transcriptomic analysis of microglia from these mice also revealed transcriptomic changes induced by R47H that had substantial overlaps with R47H microglia in human AD brains, including robust increases in proinflammatory cytokines, activation of AKT signaling, and elevation of a subset of DAM signatures.
Pharmacological AKT inhibition with MK-2206 largely reversed the enhanced inflammatory signatures in primary R47H microglia treated with TAU fibrils. In R47H heterozygous tauopathy mice, MK-2206 treatment abolished a tauopathy-dependent microglial subcluster and rescued tauopathy-induced synapse loss.
By uncovering disease-enhancing mechanisms of the R47H mutation conserved in human and mouse, our study supports inhibitors of AKT signaling as a microglial modulating strategy to treat AD.






