Summary: Sustained microglia activation leads to the cells becoming senescent. This leads to an accelerated accumulation of amyloid in the brain, influencing the early stages of Alzheimer’s development.
Source: University of Southampton
A new study has pinpointed a small group of cells in the brain which could be crucial to understanding how Alzheimer’s disease begins and how to slow its progression. This discovery could help research into treatment for the disease by focusing on this key group of cells in the brain.
Alzheimer’s disease is the most common type of dementia, affecting between 50 and 75 percent of those diagnosed. There are currently around 850,000 people with dementia in the UK. This is projected to rise to 1.6 million by 2040.
“Alzheimer’s disease evolves over decades but we currently lack an understanding of the events that take place in the early stages,” explained Dr Diego Gomez-Nicola of the University of Southampton who led this research.
The Southampton team had already discovered that microglia, the brain’s main immune cells, are the first cells to react to the build-up of a protein called amyloid, which is associated with the development of dementia, by increasing in number.
In this new study, published in the journal Cell Reports, Dr Gomez-Nicola and his team set out to explore whether this sustained encounter between microglia and amyloid would impose long-lasting changes in microglia that would influence the speed at which the disease develops.
Using a mouse model of Alzheimer’s disease-like pathology, the researchers found key evidence that the sustained activation of microglia leads to a fraction of these cells becoming senescent, a state of metabolic and inflammatory dysfunction. These senescent microglia in turn accelerated the accumulation of amyloid, impacting the early stages of the development of the disease. The findings were validated in human post-mortem samples from patients with Alzheimer’s disease.
“We have previously established that microglia respond to toxic amyloid by proliferating, which is part of their function as immune cells, they are trying to contain a foreign protein,” said Dr Gomez-Nicola.
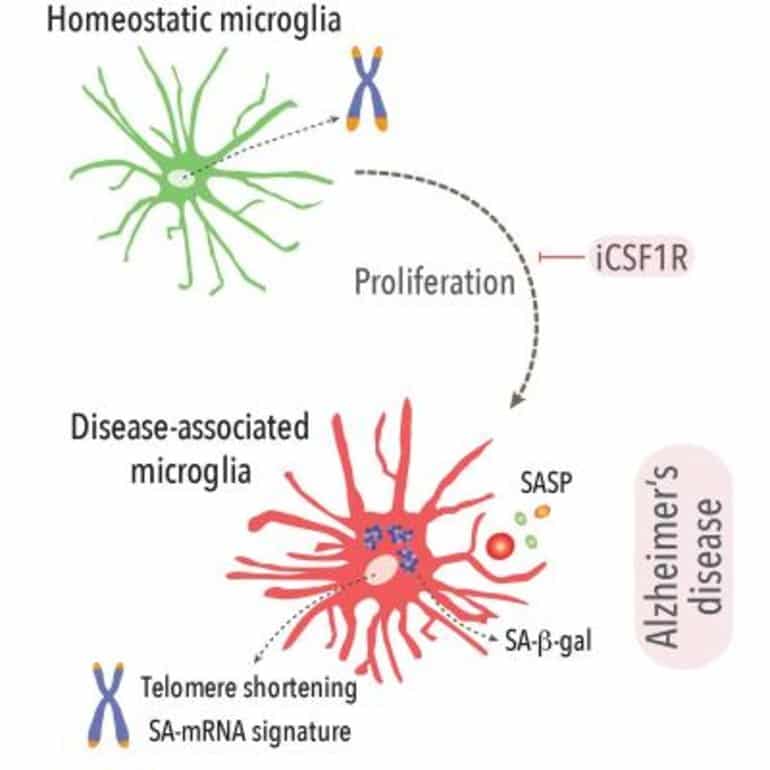
“However, this is the first time we have seen the long-term consequences of this proliferation on the cells, and the impact for the development of the disease.”
The research team then discovered that by halting the proliferation of microglia in mice, they could slow down the rate at which senescence occurred which in turned reduced the level of toxic amyloid in the brain. This could have major implications for slowing down the progression of the disease.
Dr Gomez-Nicola continued, “These findings have pinpointed a small group of microglia that have a profound influence on the rate at which Alzheimer’s disease accelerates. As well as providing scientists with more insight into the starting point of the disease, it will enable future research and drug discovery efforts to be refined to target these senescent cells specifically, and hopefully expedite further breakthroughs in the search for effective treatments.”
Researchers used the APP/PS1 mouse model for this study. All procedures were performed in accordance with UK Home Office regulations. The University of Southampton has a long-standing track record in complying with all legal requirements involving animal research and is amongst a number of organisations in the UK signed up to the Concordat on Openness on Animal Research.
About this Alzheimer’s disease research news
Source: University of Southampton
Contact: Steve Bates – University of Southampton
Image: The image is in the public domain
Original Research: Open access.
“Replicative senescence dictates the emergence of disease-associated microglia and contributes to Aβ pathology” by Diego Gomez-Nicola et al. Cell Reports
Abstract
Replicative senescence dictates the emergence of disease-associated microglia and contributes to Aβ pathology
Highlights
- •In Alzheimer’s-like pathology, a fraction of microglia undergo replicative senescence
- •Disease-associated microglia (DAM) display several features of senescence
- •Prevention of proliferation impairs the development of microglial senescence and DAMs
- •Prevention of microglial senescence leads to reduced amyloidosis and synaptic damage
Summary
The sustained proliferation of microglia is a key hallmark of Alzheimer’s disease (AD), accelerating its progression.
Here, we aim to understand the long-term impact of the early and prolonged microglial proliferation observed in AD, hypothesizing that extensive and repeated cycling would engender a distinct transcriptional and phenotypic trajectory.
We show that the early and sustained microglial proliferation seen in an AD-like model promotes replicative senescence, characterized by increased βgal activity, a senescence-associated transcriptional signature, and telomere shortening, correlating with the appearance of disease-associated microglia (DAM) and senescent microglial profiles in human post-mortem AD cases.
The prevention of early microglial proliferation hinders the development of senescence and DAM, impairing the accumulation of Aβ, as well as associated neuritic and synaptic damage.
Overall, our results indicate that excessive microglial proliferation leads to the generation of senescent DAM, which contributes to early Aβ pathology in AD.






