Worldwide, more than 20% of persons over the age of 85 suffer from Alzheimer’s disease (AD). Deposits of beta-amyloid (Aβ) peptide represent an important target for research into AD. These peptide fragments, which accumulate in the brains of AD patients, play an important role in the pathogenesis of AD. A research team headed by Dr. Frank Heppner, Director of Charité’s Department of Neuropathology, had previously demonstrated that the brain’s immune cells, known as microglia, are impaired in the course of AD. Thus, microglia in AD are unable to fulfill their primary purpose, which is the elimination of foreign substances or abnormal structures such as pathological beta-amyloid peptides.
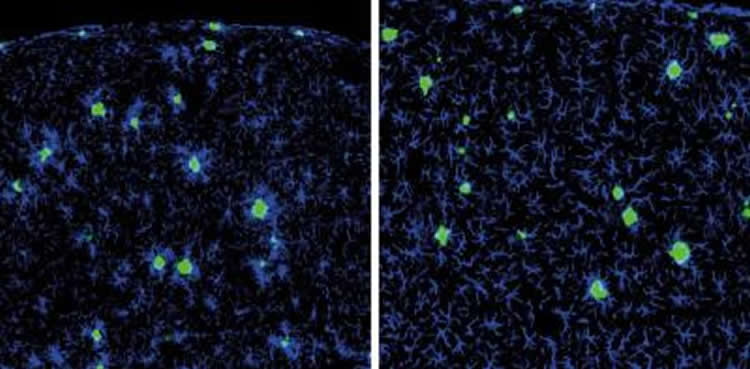
In this current study, the same team of researchers set out to investigate whether it might be possible to prompt macrophages, the peripheral counterparts of microglia that reside outside the brain and in the blood, to migrate to the brain, in order to take over role of the dysfunctional microglia. The researchers started by developing a mouse model of AD, in which they ablated microglia. This created an emergency situation, which prompted the brain to initiate an infiltration response, and resulted in blood-borne macrophages migrating from peripheral sites to repopulate the brain. These peripheral derived cells subsequently underwent further development in the brain that rendered them similar to microglia, however, without impacting AD pathology and, rather than clustering around Aβ deposits , they completely ignored their presence. These findings are mirrored by another study, which was conducted by a group of researchers from the University of Tübingen, and also published in the current volume of the Journal of Experimental Medicine.
Dr. Frank Heppner explains that “in order to make these peripheral macrophages interested in Aβ peptides, AD mice harboring blood-borne macrophages instead of resident microglia in the brain were given an Aβ vaccine – an approach that is currently being investigated in several clinical trials, and remains the subject of intense discussion.”However, even this additional stimulation did not render these peripheral macrophages any more effective than the brain’s resident microglia. “Obviously, motivating resident microglia or peripheral macrophages to realize their full potential will require a different, or additional, stimulus,” says Prof. Heppner. “However, our data is of relevance, insofar as many recent studies have identified microglia as a crucial player in both the development and progression of Alzheimer’s disease. It is therefore of fundamental importance, not least in relation to the development of new treatment options, that we should gain a detailed insight into both the role and function of microglia and macrophages in AD.”
The researchers are now planning to conduct further studies aimed at identifying the missing stimulus. By doing so, they hope to ensure that the phagocytic cells in question can return to fulfilling their original function.
Source: Dr. Frank Heppner – Charité
Image Credit: The image is credited to the researchers/JEM
Original Research: Abstract for “Impact of peripheral myeloid cells on amyloid-β pathology in Alzheimer’s disease–like mice” by Stefan Prokop, Kelly R. Miller, Natalia Drost, Susann Handrick, Vidhu Mathur, Jian Luo, Anja Wegner, Tony Wyss-Coray, and Frank L. Heppner in Journal of Experimental Medicine. Published online October 12 2015 doi:10.1084/jem.20150479
Abstract
Impact of peripheral myeloid cells on amyloid-β pathology in Alzheimer’s disease–like mice
Although central nervous system–resident microglia are believed to be ineffective at phagocytosing and clearing amyloid-β (Aβ), a major pathological hallmark of Alzheimer’s disease (AD), it has been suggested that peripheral myeloid cells constitute a heterogeneous cell population with greater Aβ-clearing capabilities. Here, we demonstrate that the conditional ablation of resident microglia in CD11b-HSVTK (TK) mice is followed by a rapid repopulation of the brain by peripherally derived myeloid cells. We used this system to directly assess the ability of peripheral macrophages to reduce Aβ plaque pathology and therefore depleted and replaced the pool of resident microglia with peripherally derived myeloid cells in Aβ-carrying APPPS1 mice crossed to TK mice (APPPS1;TK). Despite a nearly complete exchange of resident microglia with peripheral myeloid cells, there was no significant change in Aβ burden or APP processing in APPPS1;TK mice. Importantly, however, newly recruited peripheral myeloid cells failed to cluster around Aβ deposits. Even additional anti-Aβ antibody treatment aimed at engaging myeloid cells with amyloid plaques neither directed peripherally derived myeloid cells to amyloid plaques nor altered Aβ burden. These data demonstrate that mere recruitment of peripheral myeloid cells to the brain is insufficient in substantially clearing Aβ burden and suggest that specific additional triggers appear to be required to exploit the full potential of myeloid cell–based therapies for AD.
“Impact of peripheral myeloid cells on amyloid-β pathology in Alzheimer’s disease–like mice” by Stefan Prokop, Kelly R. Miller, Natalia Drost, Susann Handrick, Vidhu Mathur, Jian Luo, Anja Wegner, Tony Wyss-Coray, and Frank L. Heppner in Journal of Experimental Medicine. Published online October 12 2015 doi:10.1084/jem.20150479






