Summary: Researchers have developed brain-on-a-chip technology that uses human tissue to model how the brain’s protective barrier breaks down during inflammation and disease. The system replicates the blood-brain barrier, showing how cytokine storms and leaked blood proteins can trigger harmful changes in brain cells.
A second study revealed that pericytes, tiny support cells, can repair and stabilize damaged brain barriers—offering hope for preventing brain injury during conditions like sepsis or surgery. This innovation may one day allow doctors to test personalized drugs for neuroprotection without relying on animal models.
Key Facts:
- Inflammation Insight: Brain-on-a-chip models show that cytokine storms can cause blood-brain barrier collapse and neuronal damage.
- Pericyte Protection: Pericyte cells fill structural gaps in blood vessels, helping restore barrier integrity and brain health.
- Personalized Prevention: The chips could be used to screen neuroprotective drugs and tailor treatments before surgery or chemotherapy.
Source: University of Rochester
In lieu of animal experiments, researchers from the University of Rochester are using state-of-the-art microchips with human tissue to better understand how the brain operates under healthy conditions and is damaged through neurodegenerative diseases or conditions like sepsis.
James McGrath, the William R. Kenan Jr. Professor of Biomedical Engineering and director of the Translational Center for Barrier Microphysiological Systems (TraCe-bMPS), leads a team that develops and leverages tissue chips to study diseases where two different types of tissue meet, including at the blood-brain barrier.
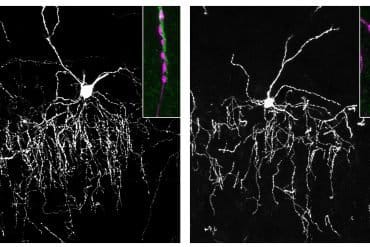
A pair of recent studies published in Advanced Science and Materials Today Bio used the chips to identify how the blood-brain barrier breaks down under serious threats, which could lead to new treatments to keep brains healthy.
When inflammation harms the brain
When a patient undergoes a major surgery or contracts an infection such as sepsis, it can excessively inflame organs throughout the body including the brain, sometimes leading to long-lasting cognitive impairment, especially in older patients.
In a study published in Advanced Science, McGrath’s team used tissue chips to show what happens at the barrier when the body suffers a cytokinetic storm—when the immune system creates an uncontrollable systemic inflammatory response. Their experiments showed that with a high enough cytokine storm, the blood-brain barrier breaks down, leading to brain injury.
“Two different stress signals—blood proteins that leak into the brain, like fibrinogen, together with inflammatory cytokines—can work together to trigger harmful changes in brain support cells called astrocytes,” says Kaihua Chen, a biomedical engineering PhD student and lead author of the study.
“At the same time, we found that the natural force of blood flow helps the blood-brain barrier stay stronger against these challenges. To me, this shows how both biology and engineering principles can come together to give us new insights into how the brain protects itself—and what goes wrong in disease.”
McGrath says that in the future, the team hopes to integrate more components of the brain on the brain side of the chip, including critical immune cells in the brain known as the microglia, to better understand how neurons are damaged during these inflammatory events. Ultimately, he hopes the chips can be used to prevent brain injuries in patients undergoing cytokine storms.
“We hope that by building these tissue models in chip format, we can arrange many brain models in a high-density array to screen candidates for neuroprotective drugs and develop brain models with diverse genetic backgrounds, including those that may be vulnerable or resilient to cytokine storms,” says McGrath.
The researchers also envision their models being used in personalized medicine, tailored to individual patients’ needs.
“If a patient is about to undergo a chemotherapy or a major surgery that risks generating cytokine storm, a chip modeling that specific patient’s brain tissue could be used to evaluate risk and guide drug choice and dosing to help prevent brain injury as an outcome,” McGrath says.
A missing key to brain health
A second study, published in Materials Today Bio, looked at pericytes, which are support cells that play an important but still not fully understood role in maintaining the blood-brain barrier. Previous studies have shown that in cases of systemic inflammation and neurodegenerative diseases, there are far fewer pericytes than in healthy brains, but it was not fully known why.
McGrath’s team engineered holes and defects in endothelial tissue—the groups of cells that form blood vessels— and introduced pericytes to see what would happen.
“It’s difficult for endothelial cells to create a proper barrier when they’re dealing with these large holes,” says McGrath. “When we add the pericytes to the membrane, they create a beautiful matrix of structural fibers that fill those holes so the endothelial cells can make their vital barrier function.”
Demonstrating the interaction between pericytes and endothelial cells opens the door to therapeutics that can preserve or introduce more pericytes to help keep the blood-brain barrier stable.
“By creating defects in the endothelial cell layer, we’re letting the cells interact more directly, allowing the pericytes to provide some of the support they do in the body,” says Michelle Trempel, a biomedical engineering PhD student and lead author of the study.
“This is important because pericyte loss is implicated in many neurodegenerative diseases, so having a model where pericytes are providing support lets us study the impact of pericyte loss in the future.”
Key collaborators on the studies included Harris (Handy) Gelbard, director of the Center for Neurotherapeutics Discovery at the University of Rochester Medical Center, Professor Niccolò Terrando from the Department of Anesthesiology at the Duke University School of Medicine, and Britta Engelhardt of the Theodor Kocher Institute at the University of Bern.
Funding: The research was supported by funding from the National Institutes of Health and a pre-doctoral fellowship from the International Foundation for Ethical Research to Kaihua Chen.
Key Questions Answered:
A: It’s a microchip that uses live human tissue to simulate brain structures like the blood-brain barrier, allowing scientists to study brain injury and disease safely and precisely.
A: Severe inflammation or cytokine storms weaken the blood-brain barrier, letting proteins and toxins leak in, which triggers harmful changes in support cells called astrocytes.
A: Pericytes help blood vessel cells repair structural damage, and their loss is linked to neurodegenerative diseases. Reintroducing or protecting them could prevent brain decline.
About this neurology and neurotech research news
Author: Luke Auburn
Source: University of Rochester
Contact: Luke Auburn – University of Rochester
Image: The image is credited to Neuroscience News
Original Research: Open access.
“Pericytes repair engineered defects in the basement membrane to restore barrier integrity in an in vitro model of the blood-brain barrier” by James McGrath et al. Materials Today Bio
Open access.
“Shear Conditioning Promotes Microvascular Endothelial Barrier Resilience in a Human BBB-on-a-Chip Model of Systemic Inflammation Leading to Astrogliosis” by James McGrath et al. Advanced Science
Abstract
Pericytes repair engineered defects in the basement membrane to restore barrier integrity in an in vitro model of the blood-brain barrier
Pericytes play a key role in the brain where they support brain microvascular endothelial cells (BMECs) in forming the tightly regulated blood-brain barrier (BBB). The loss of pericytes, and corresponding weakening of the BBB, has been reported in response to episodes of systemic inflammation and in neurodegenerative disease.
We recently demonstrated that iPSC-derived pericyte-like and BMEC-like cells form a nascent, 3D basement membrane when cultured across an ultrathin (100 nm thick) and highly nanoporous membrane (McCloskey, Ahmed et al., AHCM 2024). We also concluded that the pericyte-like cells did not contribute soluble factors to enhance permeability.
Given the structural role of pericytes in vivo, here we sought to engineer defects in the basement membrane to see if pericytes could repair them. In BMEC-like monocultures, we found that micropore (3 μm and 5 μm) patterns in nanomembranes appeared as corresponding discontinuities in basement membrane laminin and destabilized barrier function.
Both the laminin defects and the baseline barrier function were restored with the addition of pericytes on the basal side of the membrane. We further found that: 1) BMECs transmigrate through large micropores in monocultures but not in co-culture with pericytes, and 2) pericytes stabilized barrier function.
Our results align with the role of pericytes as structural support cells for the microvasculature and encourage the use of our tissue barrier platform (the μSiM) to model acute and chronic neurological disorders involving pericyte dysfunction and/or disruption of basement membrane integrity.
Abstract
Shear Conditioning Promotes Microvascular Endothelial Barrier Resilience in a Human BBB-on-a-Chip Model of Systemic Inflammation Leading to Astrogliosis
The blood–brain barrier (BBB) maintains cerebral homeostasis and protects the central nervous system (CNS) during systemic inflammation. Advanced in vitro models integrating circulation, a functional BBB, and reactive glial cells are essential for studying the link between peripheral inflammation and neuroinflammation. Fluid shear stress, a key hemodynamic parameter, strengthens microvascular barriers.
This study examines endothelial shear conditioning on barrier function in a fluidic µSiM-BBB (Microphysiological System featuring a Silicon Membrane –BBB). hiPSC-derived brain microvascular endothelial cell monocultures are conditioned with 0.5 Pa shear stress for 48 h.
Shear conditioning lowers baseline permeability, increases glycocalyx production, and reduces responses to inflammatory challenges, including barrier breakdown, ICAM-1 upregulation, and neutrophil transmigration.
Shear conditioning produces a resilient barrier function against a low-dose inflammatory challenge (10 pg mL−1 TNF-α/IL1-β/INF-γ) but a high-dose challenge (50 pg mL−1) disrupts the barrier.
Adding astrocytes as neuroinflammatory “sensors” reveals that a high-dose inflammatory challenge activates astrocytes but only in combination with fibrinogen—a plasma protein known to trigger astrogliosis in multiple neurological conditions.
This study highlights the utility of fluidic-enabled µSiM-BBB for investigating acute peripheral inflammation and brain injury relationships, serving as a foundation for more advanced models, including more cells of the neurovascular unit and brain parenchyma.