Summary: Researchers report a gut-brain neural circuit establishes the vagus nerve as an essential component of the brain system that regulates reward and motivation.
Source: Mount Sinai Hospital.
A novel gut-to-brain neural circuit establishes the vagus nerve as an essential component of the brain system that regulates reward and motivation, according to research conducted at the Icahn School of Medicine at Mount Sinai and published September 20 in the journal Cell. The study provides a concrete link between visceral organs and brain function, especially in regards to reward, and may help to inform novel targets for vagal stimulation therapy, particularly for eating and emotional disorders.
Previous research established the gut as a major regulator of motivational and emotional states but until now, the relevant gut-brain neuronal circuitry remained elusive. The vagus nerve, the longest of the cranial nerves, contains motor and sensory fibers and passes through the neck and thorax to the abdomen. Traditionally, scientists believed that the nerve exclusively mediated suppressive functions such as fullness and nausea; in contrast, circulating hormones, rather than vagal transmission, were thought to convey reward signals from the gut to the brain.
“Our study reveals, for the first time, the existence of a neuronal population of ‘reward neurons’ amid the sensory cells of the right branch of the vagus nerve,” says Ivan de Araujo, DPhil, Senior Faculty in the Department of Neuroscience at the Icahn School of Medicine at Mount Sinai and senior author of the paper. “We focused on challenging the traditional view that the vagus nerve is unrelated to motivation and pleasure and we found that stimulation of the nerve, specifically its upper gut branch, is sufficient to strongly excite reward neurons lying deep inside the brain.”
The branches of the vagus nerve are intricately intermingled, making it extremely difficult to manipulate each organ separately. To address this challenge, the research team employed a combination of virally delivered molecular tools that allowed them to exclusively target the vagal sensory neurons connected to the stomach and upper intestine.
Specifically, researchers combined different viruses carrying molecular tools in a way that allowed them to optically activate vagal neurons connected to the gut while vagal neurons leading to other organs remained mute. The approach, a state-of-the-art technique known as “optogenetics,” allows investigators to use light to manipulate the activity of a prespecified set of neurons.
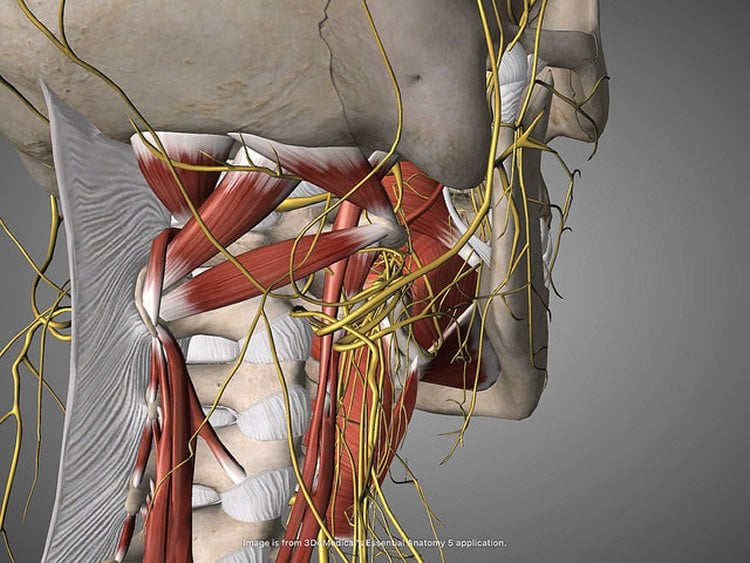
The study revealed that the newly identified reward neurons of the right vagus nerve operate under the same constraints attributed to reward neurons of the central nervous system, meaning they link peripheral sensory cells to the previously mapped populations of reward neurons in the brain. Strikingly, neurons of the left vagus were associated with satiety, but not with reward. The research team’s anatomical studies also revealed, for the first time, that the right and left vagal branches ascend asymmetrically into the central nervous system.
“We were surprised to learn that only the right vagal branch eventually contacts the dopamine-containing reward neurons in the brainstem,” explained Wenfei Han, MD, PhD, Assistant Professor of Neuroscience at the Icahn School of Medicine at Mount Sinai and lead author of the study. Dopamine is a neural transmitter known to be essential for reward and motivation.
The uncovering of right gastrointestinal vagal neurons as conveyors of reward signals to the brain opens opportunities for novel, more specific stimulation targets that may increase the efficacy of vagal nerve stimulation therapy, a treatment that involves delivering electrical impulses to the vagus nerve, for patients suffering from emotional and eating disorders.
Source: Elizabeth Dowling – Mount Sinai Hospital
Publisher: Organized by NeuroscienceNews.com.
Image Source: NeuroscienceNews.com image is credited to Beth Scupham. Licensed CC By 2.0.
Original Research: Abstract for “A Neural Circuit for Gut-Induced Reward” by Wenfei Han, Luis A. Tellez, Matthew H. Perkins, Sara J. Shammah-Lagnado, Guillaume de Lartigue, and Ivan E. de Araujo in Cell. Published September 20 2018.
doi:10.1016/j.cell.2018.08.049
[cbtabs][cbtab title=”MLA”]Mount Sinai Hospital”Gut Branches of Vagus Nerve Essential Components of Brain’s Reward and Motivation System.” NeuroscienceNews. NeuroscienceNews, 21 September 2018.
<https://neurosciencenews.com/gut-reward-motivation-9899/>.[/cbtab][cbtab title=”APA”]Mount Sinai Hospital(2018, September 21). Gut Branches of Vagus Nerve Essential Components of Brain’s Reward and Motivation System. NeuroscienceNews. Retrieved September 21, 2018 from https://neurosciencenews.com/gut-reward-motivation-9899/[/cbtab][cbtab title=”Chicago”]Mount Sinai Hospital”Gut Branches of Vagus Nerve Essential Components of Brain’s Reward and Motivation System.” https://neurosciencenews.com/gut-reward-motivation-9899/ (accessed September 21, 2018).[/cbtab][/cbtabs]
Abstract
A Neural Circuit for Gut-Induced Reward
The gut is now recognized as a major regulator of motivational and emotional states. However, the relevant gut-brain neuronal circuitry remains unknown. We show that optical activation of gut-innervating vagal sensory neurons recapitulates the hallmark effects of stimulating brain reward neurons. Specifically, right, but not left, vagal sensory ganglion activation sustained self-stimulation behavior, conditioned both flavor and place preferences, and induced dopamine release from Substantia nigra. Cell-specific transneuronal tracing revealed asymmetric ascending pathways of vagal origin throughout the CNS. In particular, transneuronal labeling identified the glutamatergic neurons of the dorsolateral parabrachial region as the obligatory relay linking the right vagal sensory ganglion to dopamine cells in Substantia nigra. Consistently, optical activation of parabrachio-nigral projections replicated the rewarding effects of right vagus excitation. Our findings establish the vagal gut-to-brain axis as an integral component of the neuronal reward pathway. They also suggest novel vagal stimulation approaches to affective disorders.