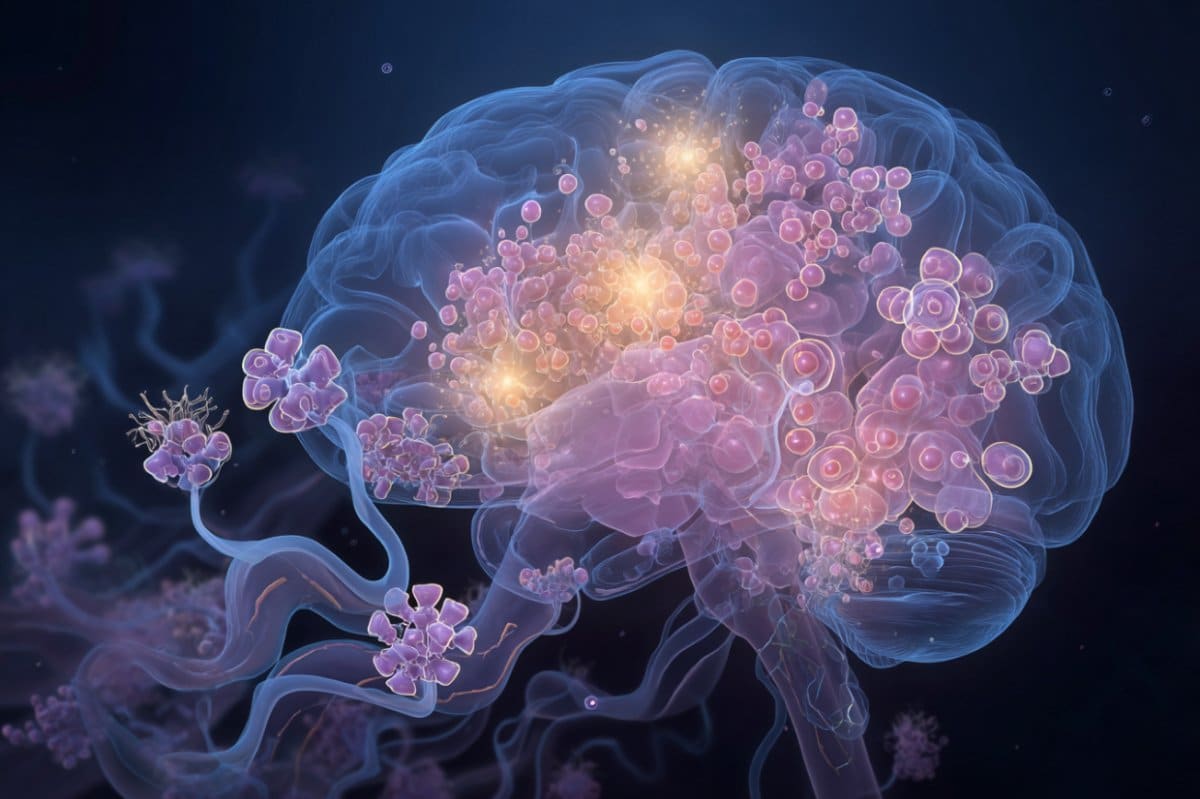
Summary: A new study reveals that an overgrowth of Candida albicans, a common gut fungus, may alter the brain’s dopamine reward circuitry and influence alcohol consumption. Researchers found that as fungal populations increased, they triggered production of inflammatory molecules called PGE2, which crossed into the brain and changed dopamine signaling in the dorsal striatum.
Unexpectedly, mice with high fungal levels began avoiding alcohol, but when PGE2 activity was blocked, they resumed drinking. The findings highlight the surprising role of fungi in the gut-brain axis and open new avenues for treating alcohol use disorder.
Key Facts:
- Fungal-Brain Link: Overgrowth of Candida albicans raised PGE2 levels that affected dopamine pathways tied to alcohol reward.
- Reversible Effect: Blocking PGE2 receptors restored alcohol preference in mice, showing a direct gut-to-brain mechanism.
- Therapeutic Potential: Understanding fungal influence on dopamine signaling could lead to new strategies for alcohol use disorder.
Source: Tufts University
Researchers at Tufts University School of Medicine and Tufts Graduate School of Biomedical Sciences have found a surprising connection between a fungus associated with alcohol use disorder and the brain’s dopamine reward pathway.
Published October 16 in the journal mBio, the study describes, in mice, how an overgrowth of Candida albicans—a fungus that naturally resides in the human gut—increases levels of inflammatory molecules called PGE2 that can cross the blood-brain barrier and affect the desire for alcohol.
PGE2, short for prostaglandin E2, is a multifunctional molecule involved in mediating inflammatory responses, reducing stomach acid, or triggering fevers. As C. albicans blooms in the gut—which is associated with antibiotic use, poor diet, or alcohol consumption—it both produces and stimulates the production of PGE2.
The study suggests that as the molecules circulate, they enter the forebrain and alter dopamine signaling in the dorsal striatum, a region involved in reward processing and habit formation.
While the researchers hypothesized that mice would find the taste of alcohol more rewarding and thus drink more when colonized with C. albicans, the results showed the opposite.
As PGE2 levels rose along with fungal populations, the mice began to avoid the beverage. When the investigators blocked PGE2 receptor molecules, the behavior was reversed, and the mice would drink alcohol again.
“Our study shows how science works—our initial ideas were very wrong,” said first author Andrew Day, who conducted the study while a Ph.D. student in the Molecular Microbiology program at the Graduate School of Biomedical Sciences.
“This could be explained by differences in how mice respond to C. albicans compared to humans, differences in fungal strains, or we might be seeing a small snapshot of the entire story.”
The researchers also discovered that mice with C. albicans overgrowth were more sensitive to alcohol’s effects on motor coordination. This effect could also be reversed by blocking PGE2 activity.
“Our bodies are wired so that our behavior responds to gut microbiota, and this study highlights that fungi are important components of the gut-brain axis,” said senior author Carol Kumamoto, a professor of molecular biology and microbiology at the School of Medicine.
“We think fungal colonization levels in individuals with alcohol use disorder could be impacting host alcohol consumption by influencing interest in drinking—whether it’s affecting how rewarding a drink may be is more of an interpretation.”
Alcohol use disorder affects over 5% of adults worldwide and is defined by an inability to control or stop alcohol consumption despite negative consequences. Traditional treatments—including behavioral therapy, support groups, medications, and maintaining abstinence—are only moderately effective, with some adults experiencing high relapse rates, creating a need for alternative approaches.
Future studies into the impact of fungi and PGE2 on alcohol use disorder could reveal new contributors to its progression. Recent clinical trials have investigated fecal microbiota transplants for the disorder, with preliminary studies showing promising effects on alcohol preference and consumption.
Additional authors are Jamie Maguire, professor at the School of Medicine, and research technician Emma Hayes of Tufts University School of Medicine; Katrina Blandino, a Ph.D. student at the Graduate School of Biomedical Sciences; Alyssa DiLeo, a graduate of the Graduate School of Biomedical Sciences; and Jeyra Perez-Lozada, formerly of Tufts University School of Medicine.
Funding:
Research reported in this article was supported by the National Institutes of Health’s National Institute of Allergy and Infectious Diseases (under award numbers T32AI007422 and R01AI118898) and National Institute on Alcohol Abuse and Alcoholism (under award number R01AA026256), and by the Tufts Initiative on Substance Use and Addiction. Complete information on authors, funders, methodology, limitations, and conflicts of interest is available in the published paper.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Key Questions Answered:
A: Overgrowth of this gut fungus increases levels of the inflammatory molecule PGE2, which can travel to the brain and alter dopamine signaling linked to alcohol reward.
A: Surprisingly, mice with high C. albicans levels drank less alcohol. When scientists blocked PGE2 signaling, the mice began drinking again.
A: The findings suggest that fungal imbalances in the gut may affect how rewarding alcohol feels, offering a new biological target for treatment.
About this AUD and neuroscience research news
Author: Rhonda Siciliano
Source: Tufts University
Contact: Rhonda Siciliano – Tufts University
Image: The image is credited to Neuroscience News
Original Research: Open access.
“Gut microbiome affects alcohol preference by influencing brain’s reward system” by Andrew Day et al. mBio
Abstract
Gut microbiome affects alcohol preference by influencing brain’s reward system
Candida albicans is a commensal yeast that is a common component of the gastrointestinal (GI) microbiome of humans. C. albicans has been shown to bloom in the GI tract of individuals with alcohol use disorder (AUD) and can promote and increase the severity of alcoholic liver disease.
However, the effects of C. albicans blooms on the host in the context of AUD or AUD-related phenotypes, such as ethanol preference, have been unstudied. In this work, we report a reduction in ethanol consumption and preference in mice colonized with C. albicans.
C. albicans-colonized mice exhibited elevated levels of serum prostaglandin E2 (PGE2), and the reduced ethanol preference was reversed by injection with antagonists of PGE2 receptors.
Furthermore, injection of mice with a PGE2 derivative decreased their ethanol preference. These results show that PGE2 acting on its receptors prostaglandin E receptor 1 (EP1) and prostaglandin E receptor 2 (EP2) drives reduced ethanol preference in C. albicans-colonized mice.
We also showed altered transcription of dopamine receptors in the dorsal striatum of C. albicans-colonized mice and more rapid acquisition of ethanol-conditioned taste aversion, suggesting alterations to reinforcement or aversion learning.
Finally, C. albicans-colonized mice were more susceptible to ethanol-induced motor coordination impairment, showing significant alterations to the behavioral effects of ethanol.
This study identifies a member of the fungal microbiome that alters ethanol preference and demonstrates a role for PGE2 signaling in these phenotypes.