Summary: A new study using SAPAP3 knockout mice sheds light on the brain mechanisms that may drive trichotillomania, or hair-pulling disorder. These mice displayed compulsive grooming, heightened aggression, and stress-sensitive behaviors, mirroring human TTM traits—particularly in females.
Neural recordings revealed reduced nucleus accumbens activity alongside dopamine receptor imbalances and altered synaptic protein interactions. The findings suggest that targeting reward circuit function and dopamine pathways could open new therapeutic avenues for compulsive hair-pulling.
Key Facts
- Circuit Dysfunction: SAPAP3 knockout mice showed reduced activity in the nucleus accumbens, a key reward and habit-control region.
- Dopamine Imbalance: Elevated dopamine and altered D1/D2 receptor expression may bias the brain toward repetitive motor behaviors.
- Complex Modulation: Oxytocin reduced aggression and grooming bouts but paradoxically increased total grooming time.
Why This Matters
- Why This Matters: Advances understanding of trichotillomania’s neurobiology, pointing to reward circuit and dopamine signaling as central drivers.
- How This Aligns with Previous Research: Builds on earlier studies linking basal ganglia dysfunction and compulsive grooming in OCD-spectrum disorders.
- Future Implications: May inform targeted treatments that balance dopamine pathways or modulate oxytocin signaling to reduce compulsive hair-pulling.
Source: Neuroscience News
Trichotillomania (TTM), or hair-pulling disorder, is an often misunderstood psychiatric condition classified among obsessive–compulsive and related disorders (OCRDs) in the DSM-5.
Affecting roughly 1.7% of adults, TTM involves recurrent urges to pull out one’s own hair, causing noticeable hair loss and significant emotional distress.
While behavioral therapies can help, pharmacological options are limited, and its neurobiological underpinnings remain incompletely understood.
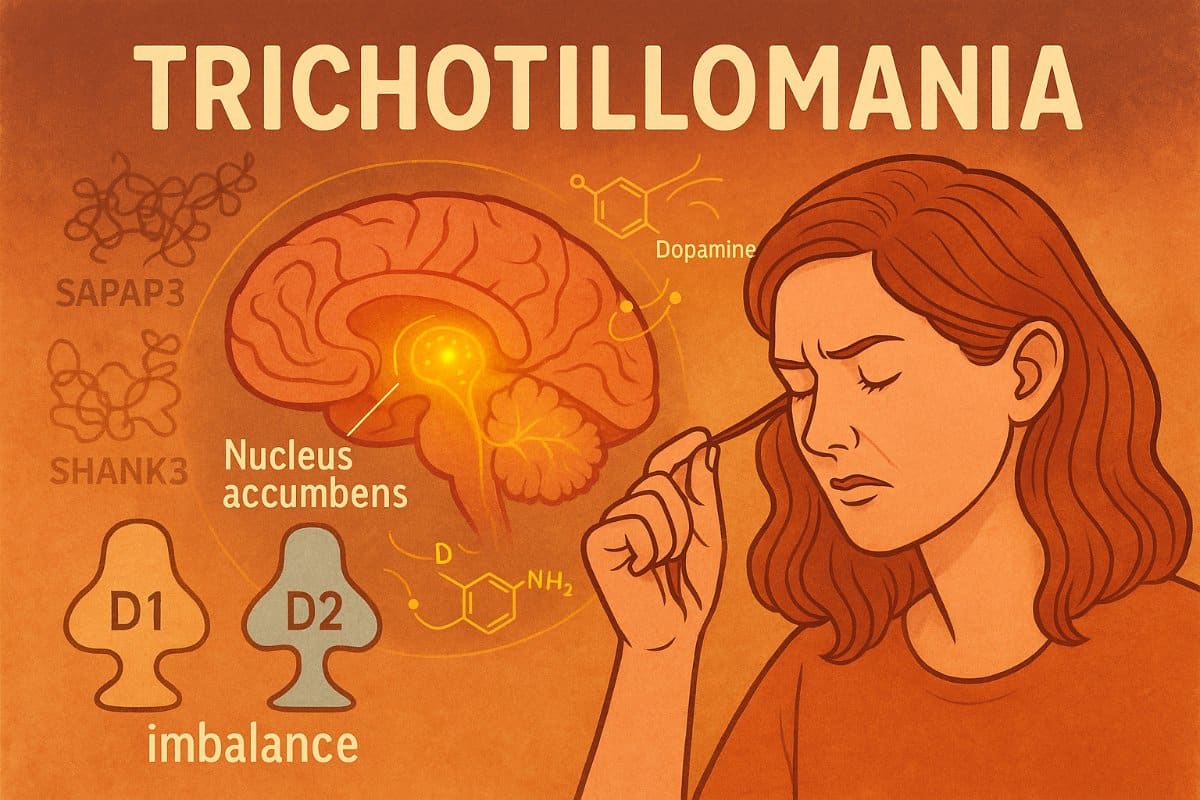
A new study using a genetically engineered mouse model lacking the Sapap3 gene—previously implicated in compulsive grooming behaviors—offers fresh insight into the brain circuitry and molecular pathways that may drive TTM.
The work also examines the surprising, sometimes paradoxical, effects of the neuropeptide oxytocin on compulsive behaviors and social aggression.
Sapap3 Knockout Mice Mirror Key Features of TTM
Sapap3 is a synaptic scaffold protein abundant in the basal ganglia, including the nucleus accumbens (NAc), a hub for reward and habit regulation. Mice lacking Sapap3 exhibited a constellation of behaviors reminiscent of human TTM: prolonged grooming under stress, increased anxiety-like behavior, and heightened social aggression.
Female knockout mice groomed for even longer than males, echoing the strong female predominance observed in clinical TTM.
Detailed behavioral assays revealed that environmental stress—in this case, aversive bright light—exacerbated these repetitive grooming patterns. In social dominance tests, Sapap3-deficient mice frequently displaced their wild-type counterparts, showing an aggressive, dominant edge under challenge.
Dopamine Imbalance and Circuit Hypoactivity
To probe the neural basis of these behaviors, the researchers used fiber-optic calcium imaging to monitor NAc activity during grooming. Sapap3 knockout mice showed markedly reduced neuronal calcium peaks compared to controls, indicating lower activity levels in this key reward-processing hub.
Molecular analysis pointed to a dopamine signaling imbalance: elevated dopamine levels, increased D1 receptor expression, and reduced D2 receptor expression in the NAc. This pattern suggests an overactivation of the “direct” pathway in medium spiny neurons, potentially biasing the brain toward repetitive, reward-seeking motor behaviors. Levels of the transcription factor CREB—known to amplify stress-related responses—were also elevated.
The team further identified a disruption in the interaction between SAPAP3 and SHANK3, another postsynaptic scaffold protein. Interestingly, SHANK3 levels rose in knockout mice, possibly as a compensatory mechanism to maintain synaptic stability.
Oxytocin’s Complex Role
Given oxytocin’s reputation for modulating social behavior and anxiety, the researchers tested its effects in Sapap3 knockout mice. The results were complex: a single dose reduced the number of grooming bouts and lessened aggression, but paradoxically increased the total time spent grooming.
This dual effect underscores the hormone’s context- and dose-dependent influence on brain circuits.
Implications for TTM Research and Treatment
This study refines our understanding of TTM’s neurobiology by highlighting NAc circuit dysfunction, dopamine receptor imbalance, and synaptic protein interactions as potential drivers of compulsive hair-pulling.
It also raises important questions about sex-specific vulnerability, the functional role of SAPAP3–SHANK3 coupling, and whether modulating oxytocin pathways could be therapeutically beneficial—or counterproductive—depending on the treatment strategy.
The work’s limitations—including small sample sizes, male-skewed molecular analyses, and the need for chronic treatment studies—leave room for future research. But by integrating behavioral, molecular, and circuit-level findings, it moves the field closer to targeted, biologically informed interventions for TTM.
About this neuroscience and trichotillomania research news
Author: Neuroscience News Editorial Team
Source: Neuroscience News
Contact: Neuroscience News Editorial Team – Neuroscience News
Image: The image is credited to Neuroscience News
Original Research: Open access.
“Exploring the nucleus accumbens circuit and oxytocin therapy in a Sapap3 knockout mouse model of trichotillomania” by Yuan Wang et al. Scientific Reports
Abstract
Exploring the nucleus accumbens circuit and oxytocin therapy in a Sapap3 knockout mouse model of trichotillomania
Trichotillomania (TTM), an understudied psychiatric disorder, was investigated using Sapap3 knockout (KO) mice to elucidate nucleus accumbens (NAc) circuit dysfunction and oxytocin’s therapeutic potential.
Under aversive conditions, KO mice exhibited TTM-like behavioral phenotypes compared to wild-type (WT) controls: elevated anxiety-like behavior (reduced total distance 33328.45 ± 6703.97 mm vs. WT 47787.22 ± 12221.33 mm; decreased standing episodes 34.20 ± 19.41 vs. WT 58.10 ± 15.55; increased immobility duration 175.05 ± 54.46 s vs. WT 90.23 ± 70.22 s, all p < 0.05), excessive grooming duration (467.43 ± 94.98 s vs. WT 391.62 ± 86.44 s, p < 0.05), and impaired social interaction characterized by elevated aggression (70 ± 10% victory rate in tube-dominance test vs. WT, p < 0.05).
Calcium imaging revealed NAc neuronal hypoactivity (peak ΔF/F0: 2.44 ± 1.67% vs. WT 6.92 ± 2.08%, p < 0.05). Molecular analyses revealed: (1) Dopaminergic signaling alterations (increased dopamine: 26.95 ± 2.04 pg/mL vs. WT 22.43 ± 1.85 pg/mL, p < 0.05; D1 receptor up-regulation 1.29-fold and D2 down-regulation 0.89-fold).
(2) Synaptic plasticity disruptions (CREB overexpression: 1.71-fold, p < 0.01; SHANK upregulation: 1.18-fold, p < 0.05). (3) SAPAP3-SHANK3 interaction deficits confirmed by immunofluorescence (compensatory SHANK3 up-regulation 1.46-fold).
Oxytocin effects were paradoxical: acute administration exacerbated total grooming duration (550.45 ± 33.65 s vs. KO baseline 467.43 ± 94.98 s, p < 0.05) but reduced grooming bouts (50.80 ± 28.20 vs. 95.30 ± 31.92, p < 0.01) and attenuated aggression (victory rate against WT decreased to 65 ± 5%).
Sex-stratified analysis revealed enhanced grooming severity in female KO mice (the grooming duration 526.59 ± 25.69 s vs. male KO 408.26 ± 104.33 s, p < 0.05).
These findings highlight NAc circuit dysfunction and complex oxytocin effects in TTM, suggesting therapeutic targets while emphasizing the need for sex-stratified.






