Summary: D-Cycloserine, an antibiotic used for the treatment of tuberculosis, increases the effectiveness of transcranial magnetic stimulation for those with major depressive disorder.
Source: University of Calgary
University of Calgary researchers have shown that the antibiotic D-Cycloserine (DCS) increases the effectiveness of transcranial magnetic stimulation (TMS) for people with major depressive disorder (MDD).
TMS is a non-invasive, well-recognized therapy for people who have treatment resistant depression. Even so, it doesn’t work for everyone. Researchers suspect the problem may be connected to a process in the brain essential for learning and memory.
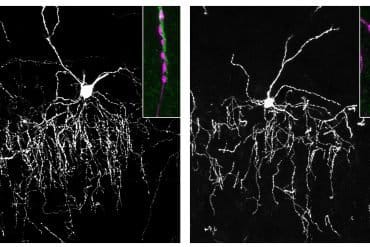
“We think TMS works by driving the brain to adapt to stimulation through a process called synaptic plasticity,” explains Dr. Alexander McGirr, MD, PhD, principal investigator on the study.
“One of the challenges, however, is that major depression is associated with reduced synaptic plasticity, and so TMS may be asking the depressed brain to adapt to stimulation in a way that it can not readily do. Adding D-Cycloserine to the TMS treatment appears to enhance TMS’s ability to drive synaptic plasticity and treat depression.”
All participants in the study underwent TMS every day for four weeks. Half of those also received DCS while the other half received a placebo. Results, published in JAMA Psychiatry, show that almost 75 percent of participants treated with DCS and TMS benefitted, compared to only 30 percent of those treated with TMS and a placebo. Depressive symptom severity was measured using the gold standard Montgomery Asberg Depression Rating Scale.
“The combination treatment seemed to have benefits beyond depressive symptoms. The participants in this study that received DCS and TMS also had greater improvements in their symptoms of anxiety and overall well-being,” says study first author Jaeden Cole a member of the McGirr lab.

The clinical trial involved 50 people. McGirr’s team plans to duplicate the research method with a larger group to be sure of the clinical efficacy and safety of this experimental treatment.
“It is hard to convey how important this work could be for patients or the level of excitement that has been brewing since Dr. McGirr first presented these results,” says Dr. Valerie Taylor, MD, PhD, head of the Department of Psychiatry at the Cumming School of Medicine.
“If confirmed, this could change practice and have a very significant impact on patients’ treatment outcomes.”
DCS is still used in the treatment of multidrug resistant tuberculosis and has been researched in other psychiatric applications such as trauma, and anxiety-related disorders. While the drug is not currently available in Canada, McGirr believes additional research proving the benefit of this combined therapy could pave the way for the drug’s reintroduction here.
Alexander McGirr has a provisional patent filing for the combination of DCS and TMS in the treatment of depression.
About this brain stimulation and depression research news
Author: Kelly Johnston
Source: University of Calgary
Contact: Kelly Johnston – University of Calgary
Image: The image is in the public domain
Original Research: Closed access.
“Efficacy of Adjunctive D-Cycloserine to Intermittent Theta-Burst Stimulation for Major Depressive Disorder A Randomized Clinical Trial” by McGirr et al. JAMA Psychiatry
Abstract
Efficacy of Adjunctive D-Cycloserine to Intermittent Theta-Burst Stimulation for Major Depressive Disorder A Randomized Clinical Trial
Importance
The antidepressant effects of transcranial magnetic stimulation protocols for major depressive disorder (MDD) are thought to depend on synaptic plasticity. The theta-burst stimulation (TBS) protocol synaptic plasticity is known to be N-methyl-D-aspartate (NMDA)–receptor dependent, yet it is unknown whether enhancing NMDA-receptor signaling improves treatment outcomes in MDD.
Objective
To test whether low doses of the NMDA-receptor partial-agonist, D-cycloserine, would enhance intermittent TBS (iTBS) treatment outcomes in MDD.
Design, Setting, and Participants
This was a single-site 4-week, double-blind, placebo-controlled, randomized clinical trial conducted from November 6, 2019, to December 24, 2020, including 50 participants with MDD. Participants were recruited via advertisements and referral. Inclusion criteria were as follows: age 18 to 65 years with a primary diagnosis of MDD, a major depressive episode with score of 18 or more on the 17-item Hamilton Depression Rating Scale, a Young Mania Rating Scale score of 8 or less, and normal blood work (including complete blood cell count, electrolytes, liver function tests, and creatinine level).
Interventions
Participants were randomly assigned 1:1 to either iTBS plus placebo or iTBS plus D-cycloserine (100 mg) for the first 2 weeks followed by iTBS without an adjunct for weeks 3 and 4.
Main Outcomes and Measures
The primary outcome was change in depressive symptoms as measured by the Montgomery-Åsberg Depression Rating Scale (MADRS) at the conclusion of treatment. Secondary outcomes included clinical response, clinical remission, and Clinical Global Impression (CGI) scores.
Results
A total of 50 participants (mean [SD] age, 40.8 [13.4] years; 31 female [62%]) were randomly assigned to treatment groups: iTBS plus placebo (mean [SD] baseline score, 30.3 [4.2]) and iTBS plus D-cycloserine (mean [SD] baseline score, 30.4 [4.5]). The iTBS plus D-cycloserine group had greater improvements in MADRS scores compared with the iTBS plus placebo group (mean difference, −6.15; 95% CI, −2.43 to −9.88; Hedges g = 0.99; 95% CI, 0.34-1.62). Rates of clinical response were higher in the iTBS plus D-cycloserine group than in the iTBS plus placebo group (73.9% vs 29.3%), as were rates of clinical remission (39.1% vs 4.2%). This was reflected in lower CGI-severity ratings and greater CGI-improvement ratings. No serious adverse events occurred.
Conclusions and Relevance
Findings from this clinical trial indicate that adjunctive D-cycloserine may be a promising strategy for enhancing transcranial magnetic stimulation treatment outcomes in MDD using iTBS requiring further investigation.
Trial Registration
ClinicalTrials.gov Identifier: NCT03937596